ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2010; 6(7):834-844. doi:10.7150/ijbs.6.834 This issue Cite
Review
Microbial and Bioconversion Production of D-xylitol and Its Detection and Application
1. Biorefining Research Initiative and Department of Biology, Lakehead University, Thunder Bay, ON, P7B 5E1, Canada
2. State Key Laboratory for Agrobiotechnology and College of Biological Sciences, China Agricultural University, Beijing, 100193, P. R. China
3. Department of Chemistry, Lakehead University, Thunder Bay, ON, P7B 5E1, Canada
Abstract
D-Xylitol is found in low content as a natural constituent of many fruits and vegetables. It is a five-carbon sugar polyol and has been used as a food additive and sweetening agent to replace sucrose, especially for non-insulin dependent diabetics. It has multiple beneficial health effects, such as the prevention of dental caries, and acute otitis media. In industry, it has been produced by chemical reduction of D-xylose mainly from photosynthetic biomass hydrolysates. As an alternative method of chemical reduction, biosynthesis of D-xylitol has been focused on the metabolically engineered Saccharomyces cerevisiae and Candida strains. In order to detect D-xylitol in the production processes, several detection methods have been established, such as gas chromatography (GC)-based methods, high performance liquid chromatography (HPLC)-based methods, LC-MS methods, and capillary electrophoresis methods (CE). The advantages and disadvantages of these methods are compared in this review.
Keywords: D-xylitol, Bioconversion production, Detection methods, Saccharomyces cerevisiae, Candida.
Introduction
D-Xylitol is a five-carbon polyol (five-carbon sugar alcohol), which has the capacity to form complexes with certain cations, including Cu2+, Ca2+, and Fe2+ [1]. It displaces water molecules from these metal ions and the hydration layer of proteins [2,3]. In nature, D-xylitol is found in various fruits and vegetables, such as berries, corn husks, oats, lettuces, cauliflowers, and mushrooms. It can be extracted from birch, raspberries, plums and corn fiber and so on. The content of D-xylitol in fruits and vegetables is usually low, and thus it is uneconomical to extract large amounts of D-xylitol from such sources. In industrial scale production, hemicellulose is utilized as the material to separate pure D-xylose, which is subsequently reduced to D-xylitol.
D-Xylitol has attracted worldwide interest because of its unique properties and huge potential. It has almost the same sweetness as sucrose, but lower energy value than sucrose (2.4 cal/g vs. 4.0 cal/g) [4], thus it has been used as a sugar substitute in dietary foods, especially for insulin-deficiency patients. Due to its anticariogenicity, tooth rehardening and remineralization properties, D-xylitol has been widely applied in the odontological industry. It could also prevent ear and upper respiratory infections and benefit pregnant and nursing women. According to the current price of D-xylitol of $4 - 5 kg-1 [5], the global market is about $340 million year-1, and it will definitely grow bigger and bigger.
Production of D-xylitol and screening of D-xylitol-producing microorganisms
D-xylitol is industrially produced by the chemical reduction of D-xylose derived mainly from photosynthetic biomass hydrolysates. Photosynthetic biomass is the most abundant renewable resources in the world, consisting of cellulose, hemicellulose, lignin and a low quantity of pectin, protein, extractives, and ash (Fig. 1). Hemicellulose is the second most abundant polysaccharide in nature, representing 19 - 34% of the photosynthetic biomass, just next to the most abundant biopolymer: cellulose (34 - 50%). Hemicellulose, a good resource for producing D-xylitol, is composed of D-glucose, D-galactose, D-mannose, D-xylose, D-arabinose, and D-glucuronic acid with acetyl side chains. In biomass materials, the three major components (cellulose, hemicellulose and lignin) are strongly intermeshed and chemically bound by non-covalent force or covalent cross-linkages [6]. In order to obtain pure fermentable sugar, D-xylose, from the complicated structure of biomass, it is necessary to pre-treat those materials by chemical [7] or biological hydrolysis methods [8]. In 1970, the industrial scale chromatographic method was developed in Finland, and pure D-xylose was separated from hemicellulose. Subsequently, D-xylitol could be produced from D-xylose through catalytic hydrogenation, in the presence of a nickel catalyst at high temperature (80 - 140oC) and pressure (up to 50 atm) [9]. The conversion rate of xylan (a polymer of D-xylose) to D-xylitol reached 50% - 60% [10].
Production of D-xylitol from photosynthetic biomass.
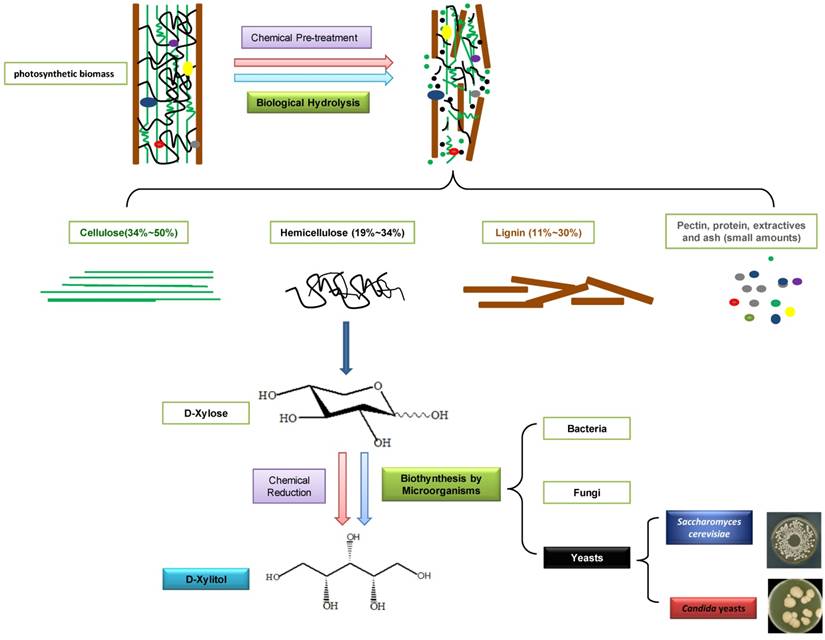
With the increase of interest in exploring more environment-friendly and economical D-xylitol production methods, the biosynthesis of D-xylitol using microorganisms recently became increasingly popular. A considerable number of bacteria, fungi and yeasts can produce D-xylitol. In Table 1, we list ten D-xylitol producing microorganisms which have been used. Corynebacterium sp. produced 69 mg/ml of D-xylitol after 14 days of incubation [11]. Enterobacter liquefaciens, which was isolated from soil, could yield 33.3 mg/ml of D-xylitol when D-xylose was used as a single carbon source [12, 13]. When grown in anaerobic conditions, Mycobacterium smegmatis could transform 70% of D-xylulose to D-xylitol [14]. Petromyces albertensis yielded 39.8 g/L of D-xylitol, in the presence of 100 g/L of D-xylose in the medium supplemented with 1% (v/v) methanol [15]. Among the microorganisms, yeasts were the preferred producer. In 1981, Barbosal et al. screened 44 yeast strains, among which the best D-xylitol producers were Candida guilliermondii and C. tropicalis [16]. A mutant strain, C. tropicalis HXP2, was reported to yield more than 90% D-xylitol from D-xylose [17]. Guo et al. (2006) tested five D-xylitol-producing strains from 274 strains [18]. Two of them, C. guilliermondii Xu280 and C. maltosa Xu316 had the highest ability to consume D-xylose and produce D-xylitol in the batch fermentation with micro-aerobic condition [18]. Suryadi et al. found that after 4 days of cultivation, Hansenula polymorpha could produce 58 g/L of D-xylitol, using 125 g/L of D-xylose in the medium [19]. Sampaio et al. (2008) screened approximately 270 yeasts for D-xylitol production using D-xylose as the sole carbon source. The best producer was Debaryomyces hansenii UFV-170, which produced 5.84 g/L of D-xylitol from 10 g/L D-xylose after 24h incubation [20] (Table 1).
Microbial conversion of D-xylose to D-xylitol
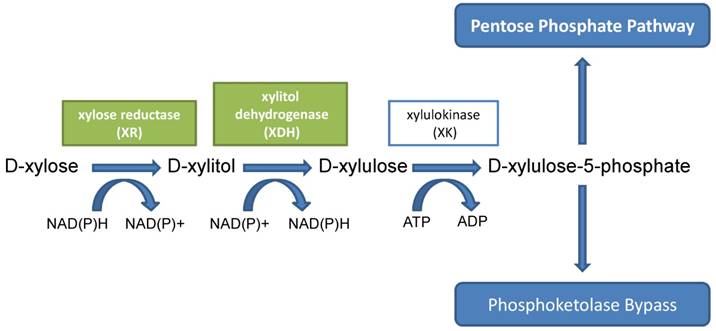
In bacteria, the conversion of D-xylose to D-xylulose is catalyzed by xylose isomerase in a single step. This xylose isomerase was also detected in some yeasts and molds, such as C. boidinii, Malbranchea pulchella, and Meurospora crassa [21-23]. However, in the majority of yeasts and fungi, the conversion of D-xylose to D-xylulose needs two steps, a reduction step followed by an oxidation step. In these yeasts and fungi, D-xylose was first reduced to D-xylitol by either NADH- or NADPH-dependent xylose reductase (aldose redutase EC 1.1.1.21) (XR); the resulting D-xylitol was either secreted or further oxidized to D-xylulose by NAD- or NADP-dependent xylitol dehydrogenase (EC 1.1.1.9) (XDH). These two reactions were considered to be the rate-limiting steps in D-xylose fermentation and D-xylitol production. Some strains of yeast could metabolize D-xylulose to xylulose-5-phosphate by xylulokinase (EC 2.7.1.17) (XK). Xylulose-5-phosphate can subsequently enter the pentose phosphate pathway [24, 25] (Fig. 2).
Screening of microorganisms for D-xylitol production.
| Strians | Carbon Sources | Growth Conditions | Yield | References |
|---|---|---|---|---|
| Corynebacterium sp. | D-xylose | ─ | 69 mg/ml | 11 |
| Enterobacter liquefaciens | D-xylose | ─ | 33.3 mg/ml | 12, 13 |
| Mycobacterium smegmatis | D-xylulose, xylitol or D-mannitol | Anaerobic condition | 0.7 g/g | 14 |
| Petromyces albertensis | D-xylose and methanol | Initial pH of 7.0 | 39.8 g/L | 15 |
| C. guilliermondii FTI-20037 | D-xylose | Aerobic condition, 30~35℃ | 77.2 g/L | 16 |
| C. tropicalis HXP2 | D-xylose | Aerobic condition, 30℃ | 0.96 g/g | 17 |
| C. guilliermondii Xu280 | D-xylose | Micro-aerobic condition | 0.63 g/g | 18 |
| C. maltosa Xu316 | D-xylose | Micro-aerobic condition | 0.43 g/g | 18 |
| Hansenula polymorpha | D-xylose and glycerol | pH of 8 | 0.52 g/g | 19 |
| Debaryomyces hansenii UFV-170 | D-xylose | Micro-aerobic condition | 0.54 g/g | 20 |
Metabolic pathway of D-xylitol in yeasts.
The wild type Saccharomyces cerevisiae was known to be a non-xylose-fermenting yeast because of its lacking the D-xylose metabolic pathway [26]. Nevertheless, its GRAS status (generally recognized as safe) and strong tolerance to inhibitors present in lignocellulose hydrolysates attracted researchers' attention. They constructed the recombinant S. cerevisiae strain, by introducing xylitol metabolism requisite genes into it. In 1991, xylose reductase gene (XYL1) cloned from Pichia stititis CBS 6054 was transformed into S. cerevisiae under the control of the phosphoglycerate kinase (PGK) promoter. In this recombinant strain, the conversion ratio of D-xylose to D-xylitol reached over 95% [27]. Some other researchers tried to use various strategies to express xylose reductase gene derived from different strains. A xylose reductase gene from P. stipitis was expressed in S. cerevisiae in 2000. The recombinant strain could produce 0.95 g of D-xylitol from 1 g of D-xylose in the presence of glucose used as a co-substrate for co-factors regeneration [28]. Handumrongkul et al. (1998) cloned a xylose reductase gene from C. guilliermondii ATCC 20118 and expressed the gene under the control of an alcohol oxidase promoter (AOX1) in methylotrophic yeast P. pastoris. The resulting strains were able to utilize D-xylose and accumulate D-xylitol. In particular, when grown in aerobic conditions, it could produce the maximum amount of D-xylitol (7.8 g/L) [29]. Chung et al. (2002) reported that a xylose reductase gene was integrated into the chromosome of S. cerevisiae by constructing two different vectors. The recombinant strains could produce 0.90 g of D-xylitol per gram of D-xylose [30]. During the continuous D-xylose metabolic process, xylose reductase (XR) activity was not the pivotal factor which controlled the conversion [31, 32]. However, the deficiency of the key co-factor NAD(P)H broke the redox balance in the cell, thus the production of D-xylitol could not be increased further in the recombinant S. cerevisiae.
The effect of co-substrates on D-xylitol production by S. cerevisiae was evaluated. Co-substrates were necessary for growth by supplying the metabolic maintenance energy and generating the reduced co-factors. It was proved that D-glucose, D-mannose and D-fructose had high affinity with the transport system for D-xylose, and inhibited D-xylose conversion by 99%, 77% and 78%, respectively, due to the competitive relationship [32]. However, D-maltose and D-galactose had their own special transport systems. There was no inhibition of D-xylose metabolism with D-maltose and 51% inhibition with D-galactose. More than 5 times higher D-xylitol production was obtained in the presence of D-galactose than D-glucose. The differences in D-xylitol yield observed with various co-substrates were hypothesized to be due to the differences in redox metabolism [32].
The Candida yeasts were considered as better potential candidates than the metabolically engineered S. cerevisiae, due to their characteristics: natural D-xylose consumers and maintaining the reduction-oxidation balance during D-xylitol accumulation. However, its application was limited in food industry because of the opportunistic pathogenic nature of some Candida spp. Reports about metabolic engineering methods in Candida strains are rare: Using the Ura-blasting method, two copies of xyl2 gene which encodes the xylitol dehydrogenase (XDH) in the diploid yeast C. tropicalis were sequentially disrupted. The conversion of D-xylose to D-xylitol reached 98% when glycerol was utilized as a co-substrate [33]. The C. parapsilosis xyl1 gene encoding xylose reductase (XR) which is controlled by an alcohol dehydrogenase promoter was introduced into C. tropicalis. This recombinant yeast exhibited higher D-xylitol yield than the wild type strain [34]. In optimizing the D-xylitol-fermenting conditions, such as aeration, temperature and pH, the oxygen availability was the key parameter for D-xylitol production from D-xylose. In transient oxygen limited condition, a surplus of NADH inhibited the activity of xylitol dehydrogenase, which resulted in D-xylitol accumulation [35]. In general, the optimal temperature and pH for D-xylitol-yielding yeasts was 30 - 37 oC and 4 - 6, respectively [36].
The applications of D-xylitol
As an effective and safe tooth-decay-preventive agent, D-xylitol is used in chewing gums, mouth rinse [37] and toothpaste [38, 39]. Streptococcus mutans is most notably associated with human dental decay, by attachment to the acquired enamel pellicle and direct interaction with the salivary components. The most important steps in the development of dental caries are the adherence of S. mutans to tooth surfaces and the formation of dental plaque [40]. These bacteria are known to be agglutinated by the whole saliva. The salivary agglutinating factor is a high-molecular-weight glycoprotein which occurs optimally between pH 5.0 - 7.5 [41,42]. S. mutans possess the ability to produce large amounts of intercellular polysaccharides from sucrose, which could be converted to lactic acid after prolonged incubation and markedly facilitate the colonization of S. mutans [43]. However, S. mutans cannot utilize D-xylitol. After people take D-xylitol-containing products, the lactic acid production from fermentation by these strains will be decreased. Saliva with D-xylitol is more alkaline than that containing other sugar products. When pH in the mouth rises above 7, calcium and phosphate salts in saliva start to precipitate into the parts of enamel where they are lacking. At the same time, D-xylitol increases the potential of saliva in buffering the acid in plaque [44]. D-Xylitol is able to reduce the ability of S. mutans to adhere to plaque, making it more easily removed from the plaque. In 1989, adults of mean age 22.5 years consumed 10.9 g of D-xylitol daily in chewing gum. The plaque of the subjects decreased and showed an obvious ability to resist pH drops induced by the sucrose rinse [45]. In 1995, sixty 11 to 15 years old children who wore fixed orthodontic appliances were given chewing gums containing D-xylitol. The fresh and dry weight of the dental plaque, collected at baseline and 28 days from incisors, canines and premolars from the area between gingival margin and the bracket, reduced significantly by 43% to 47%. The plaque and saliva levels of S. mutans were reduced by 13% to 33% in children receiving D-xylitol gum [46]. A 40-month double blind cohost study on the relationship between the use of chewing gum and dental caries was performed from 1989 - 1993 in Belize, Central America. The results showed that the D-xylitol-containing gum was effective in reducing caries rates and the most effective agent was a 100% D-xylitol pellet gum [47]. Makinen et al. (2001) reported the effect of a 2-month usage of saliva-stimulating pastils containing erythritol or D-xylitol. In D-xylitol-group, the mean weight of total plaque mass was reduced significantly; the plaque and salivary levels of S. mutans and plaque levels of total streptococcus were reduced significantly as well [48]. Milgrom et al. suggested the effective dose of D-xylitol was between 6.44 g/day and 10.32 g/day [49].
It was found that regular use of D-xylitol in chewing gums or syrup prevented the incidence of acute otitis media (AOM) in children [50]. D-Xylitol had the ability to reduce the growth of the major otopathogen of acute otitis media, S. pneumonia, which caused 30% or more of such attacks, and also suppressed Haemophilus influenza, another important pathogen implicated in AOM [51]. Kontiokari et al. reported that the exposure of either epithelial cells or pneumococci or both to 5% D-xylitol reduced the adherence of pneumococci. Some researchers implied that the inhibition of pneumonia growth induced by D-xylitol was mediated via the fructose phosphotransferase system. However the mechanism remains a matter for speculation [52].
In healthy humans, D-xylitol is metabolized to glucose-6-phosphate through an insulin-independent pathway in the liver and red blood cells. It is a very slow metabolism process from D-xylitol to D-glucose, so in this way the blood glucose and the insulin concentration raise gently [53]. In insulin-deficiency conditions, D-xylitol could be used as a sugar substitute. Adding the low energy content and the smaller thermogenic effects, D-xylitol appears to be an attractive alternative for non-insulin dependent diabetics [54].
Detection methods of D-xylitol
The qualitative detection of D-xylitol in different matrices is generally based on separation techniques coupled with characterization methods, and its quantification requires a standard curve of D-xylitol. The existing analytical methods for D-xylitol can be grouped into four catagories: gas chromatography (GC)-based methods, high performance liquid chromatography (HPLC)-based methods, LC-MS methods, and capillary electrophoresis methods (CE). The analysis of low molecular weight carbohydrates and sugar alcohols (including D-xylitol) by GC, HPLC and CE methods was reviewed in 2004 [55]. In this section, analytical methods for the determination of D-xylitol are briefly reviewed with emphasis on their recent developments.
1. GC and GC-MS
GC is a powerful analytical tool for the determination of volatile compounds in different matrices. Since D-xylitol is a polyol that is non-volatile, direct analysis of D-xylitol with GC is not possible. Therefore, GC analysis of D-xylitol requires pre-derivatization of the analyte, and the commonly used derivatization methods include trimethylsilylation (TMS) [56, 57] and acetylation [58, 59]. GC with flame ionization detection is one of the early methods developed for the detection of D-xylitol [59] and it is still a method of choice for the determination of D-xylitol in a complex matrix [60].
In more recent years, the coupling of GC with mass spectrometry (GC-MS) has greatly enhanced the capacity of this analytical method. GC-MS has high reproducibility, high resolution and few matrix effects [61] and has become one of the commonly used analytical approaches for the determination of D-xylitol [56, 58, 62]. The hyphenation of MS to GC has also increased the accuracy of GC methods in terms of structure determination because MS methods are able to provide structural information about the connectivity of atoms of the unknown molecule.
2. HPLC
Contrary to GC separation, HPLC does not require complicated derivatization steps of the analytes to form volatile derivatives. The HPLC method utilizes a suitable column for separation followed by specific detection of individually separated compounds. Different types of columns have been used for the separation of D-xylitol from carbohydrates and other sugar polyols, e.g., amino-based carbohydrate column [63, 64], HPX-87H organic acid column [65], TSK amide 80 column [66], and ion-exclusion column [67, 68]. A variety of detection methods, including UV detection, electrochemical detection, reflective index (RI) detection, and evaporative light-scattering (ELS) detection are available for HPLC methods. D-xylitol lacks chromophoric and fluorophoric moieties required for UV and fluorescence detection. As a result, less sensitive HPLC detection methods such as RI [65, 67, 69-71] and ELS [64] are more commonly used for the determination of D-xylitol. Detection limits and sensitivity to interference depend on the type of detector hyphenated to the HPLC separation. RI and ELS detection methods typically provide detection limits in the range of 0.05 - 1.2 μg/injection [63]. Detection methods such as pulsed amperometric detection can also be efficiently used for the detection of D-xylitol when coupled with ion chromatography, e.g., using Dionex column, and a strongly basic mobile phase (pH>12) [72].
In the study to evaluate D-xylitol producing capacity of several yeast strains isolated from different natural sources, authors first used thin layer chromatography (TLC) to rapidly identify the best producers and thereafter analyzed by HPLC with RI detection [71]. The TLC analysis was carried out on a silica gel plate with ethyl acetate: 2-propanol: water (130:57:23) as the developing solvent and stained with bromocresol green-boric acid. D-xylitol was visualized as a yellow spot on a blue background on TLC plates. A strain of C. tropicalis was found to be the most efficient D-xylitol producer in this study.
Recent development of column-switching techniques for chromatography allows the coupling of different separation modes to resolve a wide range of compounds in complicated samples [67, 73]. Cheng et al. [67] reported a column-switching HPLC technique by coupling H+ and Pb2+ ion-exclusion columns to study enzyme hydrolysis components of waste cellulosic biomass. The column-switching HPLC with RI detection was connected on-line to the immobilized enzyme reactor for successive on-line desalting and simultaneous analysis of carbohydrates in the hydrolysate of waste paper and waste tree branch by incorporating the heart-cut and the elution-time-difference techniques. D-xylitol was used as an internal standard in this study.
Since RI and ELS detection methods are of relatively low sensitivity, pre-column derivatization of D-xylitol for more sensitive HPLC detection has been investigated. Katayama et al. [66] reported a simple and sensitive pre-column HPLC method for the determination of sugar and sugar alcohols including D-xylitol in the serum. The samples were first derivatized with benzoic acid in the presence of condensing agents, 1-isopropyl-3-(3-dimethylaminopropyl) carbodiimide perchlorate (IDC) and 4-piperidinopyridine at 80oC for 60 min. The benzoylated derivatives were separated on a TSK amide 80 column and detected with a fluorescence detector at λex 275 nm and λem 315 nm. D-Xylitol was detected as its mono-benzoyl ester derivative and the detection limit of D-xylitol was 10 ng/mL. Similarly, D-xylitol was determined among other sugar alcohols after nitrobenzoylation by HPLC method with UV detection (260 nm) [74]. In this case, HPLC was performed on a phenyl column.
3. LC-MS and LC-NMR
Liquid chromatography hyphenated to mass spectrometry (LC-MS) has emerged as a popular and powerful tool for the determination of compounds in sample mixtures. Initially, GC was the only separation method able to be hyphenated to MS. However, the use of GC is restricted to a small set of molecules, i.e., those that are volatile or could be derivatized. Compared to GC-MS, sample pretreatment is usually simplified with LC-MS method by eliminating the need of derivatization. The determination of D-xylitol in atmospheric aerosols was carried out using LC-MS with positive ESI [63]. Polymer-based amino analytical columns were used to efficiently separate D-xylitol from eight other monosaccharides and sugar polyols. Isocratic elution was carried out in a mobile phase consisting of 20% of 10 mM NH4Ac aaqueous solution, 8% of methanol, and 72% of water. The [M+NH4]+ ions were found to be abundant and used for monitoring and quantification. Limit of detection was 4.7 pmol/injection for D-xylitol. In a similar study, LC-MS with negative ESI was employed to analyze sugars and sugar polyols in atmospheric aerosols [75]. Since sugars and sugar polyols lack highly acidic functional groups in their structures, their ionization through deprotonation to produce [M-H]- is not effective under either ESI or atmospheric pressure chemical ionization (APCI) without derivatization. It was found that post-column addition of chloroform in acetonitrile greatly enhanced ionization of these compounds by forming chloride adduct ion in the negative mode ESI. The detection limit of D-xylitol based on quantification of [M+35Cl]- adduct ion was 0.016 μM [75].
Proton NMR spectroscopy has become a routine tool for fast and comprehensive characterization of complex mixtures; however, due to extensive signal overlapping, the spectral complexity can be a serious hindrance when mixtures are analyzed. The development of LC-NMR, with the coupling of an HPLC step immediately prior to NMR measurement, allows compound separation and analysis to be carried out quickly [76]. LC-NMR was successfully applied for the metabolic profiling of human amniotic fluid in which more than 30 compounds including D-xylitol were identified [77]. The NMR spectrometer was equipped with a 3 mm probe head (60 μL active volume) and coupled to an ION300 ion exchange column with a mobile phase composed of 2.5 mM H2SO4 in 100% D2O. Although the LC-NMR method is a rather sophisticated analytical procedure, it provides a higher degree of accuracy in structural determination than other methods. This is because the NMR method provides structural information about the atom connectivity as well as stereochemistry of the unknown molecule.
4. Capillary electrophoresis
Capillary electrophoresis (CE) is a powerful separation technique suitable for assaying a variety of analytes in relatively complex matrices. The application of CE for the analysis of sugar polyols has been demonstrated recently in a review [55]. Since sugar polyols lack both a charge and a strong UV chromophore, CE analysis may be carried out after derivatization [78]. At the same time, the analysis of underivatized sugar polyol by CE method has been developed using high-alkaline pH to ionize the polyol making them suitable for indirect UV detection in a buffer solution. A CE method has been reported for the simultaneous analysis of underivatized acidic, neutral and amino sugars and sugar alcohols with indirect UV detection [79]. Separations are carried out on fused silica capillaries with an electrolyte consisting of 20 mM 2,6-pyridinedicarboxylic acid and 0.5 mM cetyltrimethylammonium bromide which is used to reverse the direction of electroosmotic flow. Optimun separation of carbohydrates and sugar alcohols has been achieved at pH 12.1 with the minimum detection level ranged from 23 - 71 μM for carbohydrates. Under these conditions, D-xylitol has been determined along with other 18 monosaccharides and sugar polyols. The method has been applied to the monosaccharide composion analysis in fetuin as a model glycoprotein after acid hydrolysis [79]. The advantages of the described CE method include direct analysis of monosaccharides and sugar alcohos without deriavatization and its high separation capacity for acidic, neutral, and amino sugars and sugar alcohols under a single electrophoretic condition.
For the analysis of polyols by CE method under less alkaline condition, in-situ derivatization of polyols with boric acid can be employed. Boric acid [B(OH)3] reacts readily with a diol forming borate diester complex, (RO)2BOH. The remaining hydroxyl group on the boron atom of the complex is readily ionizable, rendering the borate-complex able to migrate electrophoretically. By using on-column complexation with borate and indirect UV detection, the separation and determination of D-mannitol, D-sorbitol and D-xylitol in the form of anionic borate-polyol complexes by CE method has been demonstrated [80]. The separation is carried out in a fused silica capillary with background electrolyte of 200 mM borate buffer containing 10 mM 3-nitrobenzoate as the chromogenic co-ion. Similarly, this boric acid chemistry has been utilized in capillary isotachophoresis (ITP) for the determination of D-xylitol in multicomponent pharmaceutical formulations and foods [80, 81]. Using conductivity detection, the detection limit of D-xylitol by ITP method is 52 μM. Simplicity, accuracy, and low cost of analyses make ITP an alternative procedure to other methods described for the determination of D-xylitol.
5. Other methods
The advent of electrospray ionization has significantly increased the speed of MS analysis of complex mixtures. Direct MS method has also been used for the determination of carbohydrate compounds. In order to gain detailed information about structures of unknown molecules, Watkins et al. recently described an ESI-MS method equipped with Fourier transform ion cyclotron resonance (FT-ICR), in which ion-molecule reactions were employed for the characterization of polyols and polyol mixtures including D-xylitol [82]. Analytes introduced in the mass spectrometer were ionized by positive mode ESI and then allowed to react with the neutral reagent diethylmethoxyborane. Consecutive reactions of the hydroxyl groups of polyols resulted in products which were separated by 68 mass units in the mass spectrum, along with 30 mass shifts arising from intra-molecular derivatization of the primary derivatized products. The generated data provided structural information about the number of hydroxyl groups and their relative positions present in the unknown molecule.
Recently, Sreenath and Venkatesh [60] reported an indirect competitive immunoassay to detect and quantify D-xylitol in foods by making use of affinity-purified heptan-specific anti-D-xylitol antibodies. Reductively aminated D-xylitol-albumin conjugate has been used as the immunogen to raise IgG and IgE antibodies specific for D-xylitol [83]. The limit of detection is 1 ng for D-xylitol with this immunoassay, and the linear range of the assay for D-xylitol quantification is 5 - 400 ng. This indirect competitive ELISA may serve as a sensitive analytical tool to detect and quantify nanogram amounts of D-xylitol in various biological samples and natural/processed foods.
Biosensor technology is now widely used in the detection and control of specific compounds in fermentation broths. Takamizawa et al. [84] reported a D-xylitol biosensor composed of the partially purified D-xylitol dehydrogenase from C. tropicalis IFO 0618. When the biosensor was applied for the measurement of D-xylitol in the flow injection system, optimal operation pH and temperature were found to be 8.0 and 30 oC, respectively. The biosensor was characterized to have high affinity for NAD+ and medium affinity for D-xylitol, slow reaction time (15 min), and a narrow linear range of detection for D-xylitol (up to 3 mM).
Conclusion and future prospects
D-Xylitol is widely used in food, odontological and pharmaceutical industry due to its significant benefits on human health. Cost-effective production methods of D-xylitol are being explored. From hemicelluloses, as a component of the widespread, substantive, and inexpensive photosynthetic biomass, D-xylose could be separated and purified, and then reduced to D-xylitol. Some researchers have focused on screening for efficient D-xylitol-producing microbial strains for industry applications. Meanwhile, a large number of recombinant strains have been obtained, having improved D-xylitol yields. It is very common in yeasts to change the pathway of xylose metabolism by introducing or enhancing the xylose reductase and/or inactivating the D-xylitol dehydrogenase. In some genetically engineered Escherichia coli strains, the operon containing a set of genes responsible for the D-xylitol metabolism has been constructed in order to increase the productivity of D-xylitol. Other microorganisms such as filamentous fungus, which has the same D-xylose metabolic pathway as D-xylitol-producing yeasts, could be utilized in future for metabolically engineering modification.
There are a few analytical methods available for the detection of D-xylitol. Some analytical methods do not allow the direct determination of elemental connectivity and stereochemistry of analytes; therefore the accurate assignment of the analyte's structure may not be possible based on these data alone. In general, spectroscopic methods such as NMR, MS and X-ray crystallography, are the main analytical platforms which provide structural information about elemental connectivity and stereochemistry of unknown compounds. Thus, in many cases, it may not be sufficient to use a single analytical method for the detection of D-xylitol in a complicated matrix.
Acknowledgements
We thank Miranda M. Maki for critically reading the manuscript, and for her helpful comments and improvement of the text.
Conflict of Interests
The authors have declared that they have no conflict of interests.
References
1. Angyal SJ, Greeves D, Mills JA. Complexes of carbohydrates with metal cations. III. Conformations of alditols in aqueous solutions. Aust J Chem. 1974;27:1447-56
2. Sherry L. Displacement of water and its control of biochemical reactions. Chapters 1 and 2. London and New York: Academic Press. 1974
3. Gekko K, Satake L. Differential scanning calorimetry of unfreezable water in water-protein-polyol systems. Agric. Biol. Chem. 1981;45:2209-17
4. Russo JR. Xylitol: anti-carie sweetener?. Food Engineering. 1977;79:37-40
5. Prakasham RS, Sreenivas RR, Hobbs PJ. Current trends in biotechnological production of xylitol and future prospects. Curr. Trends Biotech. Pharm. 2009;3:8-36
6. Pérez J, Muñoz-Dorado J, Rubia T. et al. Biodegradation and biological treatments of cellulose, hemicelluloses and lignin: an overview. Int. Microbiol. 2002;5:53-63
7. Liaw WC, Chen CS, Chang WS, Chen KP. Xylitol production from rice straw hemicellulose hydrolyzate by polyacrylic hydrogel thin films with immobilized Candida subtropicalis WF79. J Biosci. Bioeng. 2008;105:97-105
8. Zhu L, O'Dwyer JP, Chang VS, Granda CB, Holtzapple MT. Structural features affecting biomass enzymatic digestibility. Bioresour Technol. 2008;99:3817-28
9. Härkönen M, Nuojua P. Eri tekijöiden vaikutus ksyloosin katalyyyttiseen hydraukseen ksylitoliksi. Kemia-Kemi. 1979;6:445-7
10. Hyvönen L, Koivistoinen P, Voirol F. Food technological evaluation of xylitol. Adv. Food Res. 1982;28:373-403
11. Yoshitake J, Ohiwa H, Shimamura M, Imai T. Production of Polyalcohol by a Corynebacterium sp Part I. Production of Pentitol from Aldopentose. Agric Biol Chem. 1971;35:905-11
12. Yoshitake J, Ishizaki H, Shimamura M, Imai T. Xylitol production by an Enterobacter species. Agric. Biol. Chem. 1973;37:2261-6
13. Yoshitake J, Shimamura M, Ishizaki H, Irie Y. Xylitol production by Enterobacter liquefaciens. Agric. Biol. Chem. 1976;40:1493-503
14. Izumori K, Tuzaki K. Production of xylitol from D-xylulose by Mycobacterium smegmatis. J Ferment. Technol. 1988;66:33-6
15. Dahiya JS. Xylitol production by Petromyces albertensis grown on medium containing D-xylose. Can. J Microbiol. 1991;37:14-31
16. Maria F, Barbosa S, de Medeiros MB, de Mancilha IM, Schneider H, Lee H. Screening of yeasts for production of xylitol fromd-xylose and some factors which affect xylitol yield in Candida guilliermondii. J Ind. Microbiol. Biotechnol. 1988;3:241-51
17. Gong CS, Chen LF, Tsao GT. Quantitative production of xylitol from D-xylose by a high-xylitol producing yeast mutant Candida tropicalis HXP2. Biotechnol. Lett. 1981;3:125-30
18. Guo C, Zhao C, He P, Lu D, Shen A, Jiang N. Screening and characterization of yeasts for xylitol production. J Appl. Microbiol. 2006;101:1096-104
19. Suryadi H, Katsuragi T, Yoshida N, Suzuki S, Tani Y. Polyol production by culture of methanol-utilizing yeast. J Biosci. Bioeng. 2000;89:236-40
20. Sampaio FC, Chaves-Alves VM, Converti A, Lopes Passos FM, Cavalcante Coelho JL. Influence of cultivation conditions on xylose-to-xylitol bioconversion by a new isolate of Debaryomyces hansenii. Bioresour Technol. 2008;99:502-8
21. Vongsuvanlert V, Tani Y. Purification and characterization of xylose isomerase of a methanol yeast, Candida boidinii, which is involved in sorbitol production from glucose. Agric. Biol. Chem. 1988;52:1817-31
22. Banerjee S, Archana A, Satyanarayana T. Xylose metabolism in a thermophilic mould Malbranchea pulchella var. sulfurea TMD-8. Curr. Microbiol. 1994;29:349-52
23. Rawat U, Phadtare S, Deshpande V, Rao M. A novel xylose isomerase from Neurospora crassa. Biotechnol. Lett. 1996;18:1267-70
24. Lachke AH, Jeffries TW. Levels of the enzymes of the pentose phosphate pathway in Pachysolen tannophilus Y-2460 and selected mutants. Enz. Microb. Technol. 1986;8:353-9
25. Smiley KL, Bolen PL. Demonstration of D-xylose reductase and xylitol dehydrogenase in Pachysolen tannophilus. Biotechnol. Lett. 1982;4:607-10
26. Gong CS, Chen LF, Flickinger MC, Chiang LC, Tsao GT. Production of ethanol from D-xylose by using D-xylose isomerase and yeasts. Appl. Environ. Microbiol. 1981;41:430-6
27. Hallborn J, Walfridsson WM, Airaksinen U, Ojamo H, Hahn-Hagerdal B, Penttila M, Keranen S. Xylitol production by recombinant Saccharomyces cereisiae. Biotechnol. 1991;9:1090-5
28. Leea WJ, Ryu YW, Seo JH. Characterization of two-substrate fermentation processes for xylitol production using recombinant Saccharomyces cerevisiae containing xylose reductase gene. Process Biochemistry. 2000;35:1199-203
29. Handumrongkul C, Ma DP, Silva JL. Cloning and expression of Candida guilliermondii xylose reductase gene ( xyl1 ) in Pichia pastoris. Appl. Microbiol. Biotechnol. 1998;49:399-404
30. Chunga YS, Kima MD, Leea WJ, Ryub YW, Kimc JH, Seoa jH. Stable expression of xylose reductase gene enhances xylitol production in recombinant Saccharomyces cerevisiae. Enz. Microb. Technol. 2002;30:809-16
31. Meinander NQ, Hahn-Hägerdal B. Fed-batch xylitol production with two recombinant Saccharomyces cerevisiae strains expressing XYL1 at different levels, using glucose as a cosubstrate: A comparison of production parameters and strain stability. Biotechnol. Bioeng. 1998;54:391-9
32. Meinander NQ, Hahn-Hagerdal B. Influence of cosubstrate concentration on xylose conversion by recombinant, XYL1-expressing Saccharomyces cerevisiae: a comparison of different sugars and ethanol as cosubstrates. Appl. Environ. Microbiol. 1997;63:1959-64
33. Ko BS, Kim J, Kim JH. Production of xylitol from D-xylose by a xylitol dehydrogenase gene-disrupted mutant of Candida tropicalis. Appl. Environ. Microbiol. 2006;72:4207-13
34. Lee JK, Koo BS, Kim SY. Cloning and characterization of the xyl1 gene, encoding an NADH-preferring xylose reductase from Candida parapsilosis, and its functional expression in Candida tropicalis. Appl. Environ. Microbiol. 2003;69:6179-88
35. Granstrom T, Ojamo H, Leisola M. Chemostat study of xylitol production by Candida guilliermondii. Appl. Microbiol. Biotechnol. 2001;55:36-42
36. Silva SS, Afschar AS. Microbial production of xylitol from D-xylose using Candida tropicalis. Bioprocess Engg. 1994;11:129-34
37. Hildebrandt G, Lee L, Hodges J. Oral mutans streptococci levels following use of a xylitol mouth rinse: a double-blind, randomized, controlled clinical trial. Special Care in Dentistry. 2010;30:53-8
38. Lif Holgerson P, Stecksen-Blicks C, Sjostrom I, Oberg M, Twetman S. Xylitol concentration in saliva and dental plaque after use of various xylitol-containing products. Caries Res. 2006;40:393-7
39. Sano H, Nakashima S, Songpaisan Y, Phantumvanit P. Effect of a xylitol and fluoride containing toothpaste on the remineralization of human enamel in vitro. J Oral Sci. 2007;49:67-73
40. Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980;44:331-84
41. Gibbons RJ, Spinell DM. Salivary induced aggregation of plaque bacteria. In: (ed.) McHugh WD. Dental plaque. Edinburgh: E & S Livingstone Inc. 1970:207-15
42. Hay DI, Gibbons RJ, Spinell DM. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5:111-23
43. Minah GE, Loesche WJ. Sucrose metabolism by prominent members of the flora isolated from cariogenic and non-cariogenic dental plaques. Infect Immun. 1977;17:55-61
44. Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986;50:353-80
45. Soderling E, Makinen KK, Chen CY, Pape HR, Loesche W, Makinen PL. Effect of sorbitol, xylitol, and xylitol/sorbitol chewing gums on dental plaque. Caries Res. 1989;23:378-84
46. Isotupa KP, Gunn S, Chen CY, Lopatin D, Makinen KK. Effect of polyol gums on dental plaque in orthodontic patients. Am. J Orthod. Dentofacial Orthop. 1995;107:497-504
47. Makinen KK, Bennett CA, Hujoel PP, Isokangas PJ, Isotupa KP, Pape HRJr, Makinen PL. Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent. Res. 1995;74:1904-13
48. Makinen KK, Isotupa KP, Kivilompolo T, Makinen PL, Toivanen J, Soderling E. Comparison of erythritol and xylitol saliva stimulants in the control of dental plaque and mutans streptococci. Caries Res. 2001;35:129-35
49. Milgrom P, Ly KA, Roberts MC, Rothen M, Mueller G, Yamaguchi DK. Mutans streptococci dose response to xylitol chewing gum. J Dental Res. 2006;85:177-81
50. Uhari M, Kontiokari T, Koskela M, Niemela M. Xylitol chewing gum in prevention of acute otitis media: double blind randomised trial. Bmj. 1996;313:1180-4
51. Kontiokari T, Uhari M, Koskela M. Antiadhesive effects of xylitol on otopathogenic bacteria. J Antimicrob. Chemother. 1998;41:563-65
52. Tapiainen T, Kontiokari T, Sammalkivi L, Ikaheimo I, Koskela M, Uhari M. Effect of xylitol on growth of Streptococcus pneumoniae in the presence of fructose and sorbitol. Antimicrob. Agents. Chemother. 2001;45:166-9
53. de Kalbermatten N, Ravussin E, Maeder E, Geser C, Jequier E, Felber JP. Comparison of glucose, fructose, sorbitol, and xylitol utilization in humans during insulin suppression. Metabolism. 1980;29:62-7
54. Natah SS, Hussien KR, Tuominen JA, Koivisto VA. Metabolic response to lactitol and xylitol in healthy men. Am. J Clin. Nutr. 1997;65:947-50
55. Martínez Montero C, Rodríguez Dodero MC, Guillén Sánchez DA, Barroso C G. Analysis of low molecular weight carbohydrates in food and beverages: a review. Chromagrophia. 2004;59:15-30
56. Namgung HJ, Park HJ, Cho IH, Choi HK, Kwon DY, Shim SM, Kim YS. Metabolite profiling of doenjang, fermented soybean paste, during fermentation. J Sci. Food Agric. 2010;90:1926-35
57. Sweeley CC, Bentley R, Mikita M, Wells WW. Gas-liquid chromatography of trimethylsilyl derivatives of sugar and related substances. J Am. Chem. Soc. 1963;85:2497-507
58. Lee J, Chung BC. Simultaneous measurement of urinary polyols using gas chromatography/mass spectrometry. J Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006;831:126-31
59. Makinen K, Soderling E. A quantitative study of mannitol, sorbitol, xylitol, and xylose in wild berries and commercial fruits. J Food. Sci. 1980;45:367-74
60. Sreenath K, Venkatesh YP. Quantification of xylitol in foods by an indirect competitive immunoassay. J Agric. Food Chem. 2009;58:1240-6
61. Kopka J. Current challenges and developments in GC-MS based metabolite profiling technology. J Biotechnol. 2006;124:312-22
62. Clayton SJ, Read DB, Murray PJ, Gregory PJ. Exudation of alcohol and aldehyde sugars from roots of defoliated Lolium perenne L. grown under sterile conditions. J Chem. Ecol. 2008;34:1411-21
63. Wan ECH, Yu JZ. Determination of sugr compounds in atmospheric aerosols by liquid chromatography combined with positive electrospray ionization mass spectrometry. J Chromatogr. A. 2006;1107:175-81
64. Bhandari P, Kumar N, Singh B, Kaul VK. Simultaneous determination of sugars and picrosides in Picrorhiza species using ultrasonic ectraction and high-performance liquid chromatography with evaporative light scattering detection. J Chromatogr. A. 2008;1194:257-61
65. Park SM, Sang BI, Park DW, Park DH. Electrochemical reduction of xylose to xylitol by whole cells or crude enzyme of Candida peltata. J Microbiol. 2005;43:451-5
66. Katayama M, Matsuda Y, Kobayashi K, Kaneko S, Ishikawa H. Simultaneous determination of glucose, 1,5-anhydro-d-glucitol and related sugar alcohols in serum by high-performance liquid chromatography with benzoic acid derivatization. Biomed. Chromatogr. 2006;20:440-5
67. Cheng C, Chen CS, Hsieh PH. On-line desalting and carbohydrate analysis for immobilized enzyme hydrolysis of waste cellulosic biomass by column-switching high-performance liquid chromatography. J Chromatogr. A. 2010;1217:2104-10
68. Ohsawa K, Yoshimura Y, Watanabe S. et al. Determination of xylitol in the human serum and saliva by ion chromatography with pulsed amperometric detection. Anal. Sci. 1986;2:165-8
69. Ling H, Cheng K, Ge J, Ping W. Statistical optimization of xylitol production from corncob hemicellulose hydrolysate by Candida tropicalis HDY-02. N Biotechnol. 2010 [Epub ahead of print]
70. Salgado JM, Caballo E. et al. Characterization of vinasses from five certified brands of origin (CBO) and use as economic nutrient for the xylitol production by Debaryomyces hansenii. Biores. Technol. 2010;101:2379-88
71. Altamirano A, Vazquez F, de Figueroa LI. Isolation and identification of xylitol-producing yeasts from agricultural residues. Folia. Microbiol. 2000;45:255-8
72. Tsai YI, Wu PL, Hsu YT, Yang CR. Anhydrosugar and sugar alcohol organic markers associated with carboxylic acids in particulate matter from incense burning. Atmosph. Environ. 2010;44:37- 47
73. Fukushima T, Mitsuhashi S, Tomiya M, Kawai J, Hashimoto K, Toyo'oka T. Determination of rat brain kynurenic acid by column-switching HPLC with fluorescence detection. Biomed. Chromatogr. 2007;21:514-9
74. Nojiri S, Taguchi N, Oishi M, Suzuki S. Determination of sugar alcohols in confectioneries by high-performance liquid chromatography after nitrobenzoylation. J Chromatogr. A. 2000;893:195-200
75. Wan EC, Yu JZ. Analysis of sugars and sugar polyols in atmospheric aerosols by chloride attachment in liquid chromatography/negative ion electrospray mass spectrometry. Environ. Sci. Technol. 2007;41:2459-66
76. Griffiths L. Optimization of NMR and HPLC conditions for LC-NMR. Anal. Chem. 1995;67:4091-4
77. Graca G, Duarte IF, B JG, Carreira IM, Couceiro AB, Domingues Mdo R, Spraul M, Tseng LH, Gil AM. Metabolite profiling of human amniotic fluid by hyphenated nuclear magnetic resonance spectroscopy. Anal. Chem. 2008;80:6085-92
78. Guttman A. Analysis of monosaccharide composition by capillary electrophoresis. J Chromatogr. A. 1997;763:271-7
79. Soga TH, Heigert DN. Simultaneous Determination of Monosaccharides in Glycoproteins by Capillary Electrophoresis. Anal. Biochem. 1998;261:73-8
80. Pospisilova M, Polasek M, Safra J, Petriska I. Determination of mannitol and sorbitol in infusion solutions by capillary zone electrophoresis using on-column complexation with borate and indirect spectrophotometric detection. J Chromatogr. A. 2007;1143:258-63
81. Herrmannova M, Krivankova L, Bartos M, Vytras K. Direct simultaneous determination of eight sweeteners in foods by capillary isotachophoresis. J Sep. Sci. 2006;29:1132-7
82. Watkins MA, Winger BE, Shea RC, Kenttamaa HI. Ion-molecule reactions for the characterization of polyols and polyol mixtures by ESI/FT-ICR mass spectrometry. Anal. Chem. 2005;77:1385-92
83. Sreenath K, Venkatesh YP. Reductively aminated D-xylose-albumin conjugate as the immunogen for generation of IgG and IgE antibodies specific to D-xylitol, a haptenic allergen. Bioconjug. Chem. 2007;18:1995-2003
84. Takamizawa K, Uchida S, Hatsu M, Suzuki T, Kawai K. Development of a xylitol biosensor composed of xylitol dehydrogenase and diaphorase. Can. J Microbiol. 2000;46:350-7
Author contact
![]() Corresponding author: Wensheng Qin, Biorefining Research Initiative and Department of Biology, Lakehead University, Thunder Bay, ON, P7B 5E1, Canada. Tel: 807-343 8840 / Fax: 807-346 7796; E-mail: wqinca
Corresponding author: Wensheng Qin, Biorefining Research Initiative and Department of Biology, Lakehead University, Thunder Bay, ON, P7B 5E1, Canada. Tel: 807-343 8840 / Fax: 807-346 7796; E-mail: wqinca
Received 2010-11-15
Accepted 2010-12-6
Published 2010-12-15
