ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2012; 8(1):150-158. doi:10.7150/ijbs.8.150 This issue Cite
Research Paper
Matrine Inhibits Pacing Induced Atrial Fibrillation by Modulating IKM3 and ICa-L
1. Department of Pharmacology (the State-Province Key Laboratories of Biomedicine-Pharmaceutics of China, Key Laboratory of Cardiovascular Research, Ministry of Education), Harbin Medical University, Harbin, Heilongjiang 150081, P. R. China
2. Department of Cardiology, the Second Affiliated Hospital of Harbin Medical University
3. Department of Pathophysiology, Harbin Medical University, Harbin, China
# These authors contributed equally to this study.
Abstract
Aim: To elucidate the protective effects of Matrine on atrial fibrillation (AF) induced by electric pacing in mice and underlying molecular and ion channel mechanisms.
Methods: AF was introduced by electric pacing in mice and the incidence and duration of AF were evaluated. Functional expression of M3 receptor (M3-R) and Cav1.2 were explored by western and Real-time PCR, action potential (AP) and the density of (IKM3) L-type calcium channel (ICa-L) were both recorded using whole-cell patch in isolated atrial cardiomyocytes.
Results: In control group, incidence and duration of AF induced by electric pacing were 50 ± 17% and 3.68 ± 1.84 s, respectively; after application of carbachol 50 µg/kg both incidence and duration of AF were significantly increased to 86 ± 24% and 65.2 ± 29.0 s. Compared with control group, pretreatment of Matrine for 15 days significantly reduced AF incidence and duration in dose-dependent manner. Atrial membrane-protein expression of M3-R was decreased and membrane Cav1.2 expression was up-regulated. In single Matrine-treated atrial cardiomyocyte the density of IKM3 was significantly decreased by 39% as well compared with control group, P < 0.05, whereas, ICa-L density of atrium was increased by 40%.
Conclusion: These data demonstrated at the first time that the anti-AF effects of Matrine may due, at least in part, to down-regulation of IKM3 density and M3-R expression and up-regulation of ICa-L density and α1C/Cav1.2 expression.
Keywords: atrial fibrillation, M3 receptor, L-type calcium channel, potassium channel, Matrine
Introduction
Atrial fibrillation (AF) is the most common tachyarrhythmia in clinics and its incidence increases with age by nearly 10% of people at age over 80 [1,2]. AF leads to severe cardiovascular morbidity and disability [3,4]. Current drug therapy for AF is somehow unsatisfied, so, a better understanding of arrhythmic mechanisms may allow for safer and more effective clinical management in patients with AF [5,6].
Both parasympathetic tone and electric remodeling of atrial ion channel play an important role in the pathophysiology of AF [7,8]. Studies from Yang's group and Wang's laboratory [9-11] demonstrated that several non-selective Muscarinic acetylcholine receptor (AchR) agonists including choline (0.1-10 mM), pilocarpine (0.1-10 µM), and tetramethylammonium (TMA) (1-10 mM) each can induce a similar novel delayed rectifier K+ current (termed as IKM3, meaning the M3-receptor-activated delayed rectifier K+ current) in dispersed cardiomyocytes from guinea-pig and canine atria. IKM3 could accelerate cardiac repolarization and shorten the effective refractory period (ERP), an effect favoring the occurrence of reentrant arrhythmias. It is well-known that M2-receptor (M2-R) and coupled K+ channel subunits are major parasympathetic constitution. In the atrium of dogs with AF induced by CHF the densities of the M3-R and IKM3 current are both robustly increased, but those of the M2-R are abrogated [12]. G-protein signaling proteins 2 (RGS) regulate atrial M3-R signaling and block the M3R signal transduction. The RGS2-/- mice have enhanced susceptibility to AF via enhancing M3-R activity [13]. These results indicate that the M3-R may play an important role in pathological condition and contribute to initiation and perpetuation of AF.
Abnormalities in intracellular Ca2+ handling may constitute key missing links in AF-initiating focal activity and AF perpetuation by rapidly firing foci and reentry [14,15]. In patients with AF, increased atrial rates enhance cardiomyocyte Ca2+ influx with each action potential (AP). To prevent potentially cytotoxic Ca2+ overload, atrial cardiomyocytes limit Ca2+ influx through accelerating ICa-L inactivation and down-regulation of mRNA encoding ICa-L, consequently resulting in decrease in both ICa-L and atrial AP duration (APD), which, in turn, relatively increase in ERP [16,17]. In clinical therapy of AF, current Ca2+ channel blockers are frequently used to reduce Ca2+ overload with the expectation that prevents arrhythmogenic remodeling, however, it actually aggregates down-regulation of ICa-L in the atrium so as to increase the risk of ventricular arrhythmia and does not show a beneficial effects on AF [18,19]. The electrical remodeling paradigm and unsatisfied therapeutic effects of current Ca2+ channel blockers in AF treatment lead us to find new efficient compound that can be used in AF clinical management without down-regulating Ca2+ channel.
Sophora flavescens Ait (SF), used as the dry root, is a traditional herb medicine found in China, Japan, and some European countries and has long been used as an anti-inflammatory and anti-cancer agents [20]. Among various alkaloids isolated from SF, Matrine (Fig.1) has been identified as the major bioactive component contributing to a variety of pharmacological effects such as hepatitis B and C [21,22], some cancers [23], and cardiac diseases [24]. In clinics, Matrine is currently used to treat cardiac arrhythmias, especially premature ventricular beats [25]. It was reported that anti-arrhythmic effect of Matrine was due to the prolongation of APD and the inhibition of K+ currents in ventricular cardiomyocytes [26]. In addition, Matrine enhances [Ca2+]i by stimulating ICa-L and exerts positive inotropic effects on electrically driven in guinea pig papillary muscles [27]. However, there is no published data regarding to the effects of Matrine on APD and ionic currents from atrial cardiomyocytes. Therefore, the present study is designed to elucidate the effects of Matrine on AF and underlying ion channel mechanisms in atrial cardiomyocytes.
Chemical structure of Matrine. (C15H24N2O, molecular weight = 248.36).
Materials and methods
Animals
Mice (18 - 22 g, SPF, the Animal Center of Harbin Medical University) were housed at 20 ± 3 ºC with 55 ± 10 % humidity, 12 h light/dark cycle, and had free access to species-specific food and tap water. All experiments were carried out according to the China Guide to the Care and Use of Experimental Animals. Experimental protocols used in the present study were pre-approved by the Institute Committee of the Animal Care of Harbin Medical University.
Chemicals
Matrine was obtained from Xian Botany Garden (Shanxi, China), and its purity was > 99% as assessed by high-performance liquid chromatography [28]. Matrine stock solution was prepared in double polished distilled water (ddH2O). 4-(2-hydroxyehtyl) piperazine-1-ethanesulfonic acid (HEPES), taurine, Na2-ATP, K asparate, collagenase (type II) were purchased from Sigma (St Louis, MO, U.S.A.).
Induction of atrial fibrillation in wild-type mice [29,30]
52 Mice were randomly divided into four groups: control group, Matrine 15 mg/kg (low dose, Ma-L), 30 mg/kg (medium dose, Ma-M), 45 mg/kg (high dose, Ma-H) pretreatment group. Matrine were administrated intravenously (iv) once daily for 15 days before experiment. Intracardiac pacing was performed in these animals by inserting an eight-electrode catheter (1.1 F, octapolar EP catheter, Science) through the jugular vein and advancing it into the right atrium and ventricle. Atrial arrhythmias were introduced by applying 6-second bursts through the catheter electrodes using the automated stimulator that was part of the data acquisition system. The cycle length (CL) in the first 6-second burst is 40 ms and decreases in each successive burst with a 2-ms decrement down to a CL of 20 ms. Then 50 µg/kg carbachol was injected through the jugular vein in model mice and the same combination of bursts was applied again at two minutes after carbachol. Successful AF was defined as a period of rapid irregular atrial rhythm lasting at least 1 second.
Electrophysiological recording
Single atrial cardiomyocytes were isolated from mouse heart by enzymatic dissociation according to the procedure previously described [31]. AP and ionic currents were studied in whole-cell patch configuration at room temperature (21 ~ 23 ºC). Only quiescent rod-shaped cells lacking membrane deformities and showing clear cross striations were studied. A small aliquot of the solution containing the isolated cells was placed in a 1.0 ml recording chamber mounted on the stage of a Nikon Diaphot inverted microscope (Nikon, Tokyo, Japan). Borosilicate glass electrodes with tip resistance of 2 ~ 5 MΩ were filled with the appropriate internal solution (in mM: 20 KCl, 110 potassium asparate, 1.0 MgCl2, 5.0 HEPES, 10 EGTA, 5.0 Na2-ATP, with pH adjusted to 7.2 with KOH). For Ca2+ current recordings, the recording electrodes were filled with appropriate internal solution (in mM: 20 CsCl, 110 Cesium asparate, 1.0 MgCl2, 5.0 HEPES, 10 EGTA, and 5.0 Na2-ATP with pH adjusted to 7.2 with CsOH). Junction potentials were zeroed before formation of the membrane-pipette seal in Tyrode's solution. Both AP and membrane currents were recorded with an Axo-patch 200B amplifier (Axon Instruments, U.S.A). After forming the whole-cell recording configuration, a capacitive current transient induced by a 10 mV step from a holding voltage of 0 mV was recorded and used for the calculation of cell capacitance. Series resistance (Rs) ranged from 4 ± 6 MΩ was compensated by 60 to 80%. For measurement of K+ currents, the bath Tyrode's solution was contained in mM: 126 NaCl, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, 0.33 NaH2PO4, 10 glucose and 10 HEPES with pH adjusted to 7.35 with NaOH. For Ca2+ measurement, the bath Tyrode's solution was contained in mM 136 Tris-HCl, 5.4 CsCl, 1.0 MgCl2, 1.8 CaCl2, 0.33 NaH2PO4, 10 glucose and 10 HEPES with pH adjusted to 7.35 with Tris-OH. The estimated voltage error attributed to uncompensated Rs (Rs x ICa-L) was below 5 mV in all performed cells in the current experiments.
Current-clamp was used to record AP of atrial cardiomyocytes triggered by a two milliseconds depolarizing stimulatory pulse. Voltage-clamp was used to record ionic currents. The recording protocols for both Ca2+ and K+ channel currents were described in the result section, respectively.
Quantitative real-time RT-PCR analysis
The SYBR Green PCR Master Mix Kit (Ambion) was used in quantification of mRNA in our study. Total RNA was isolated with the TRIzol method (Invitrogen) from mouse hearts. Quantitative real-time PCR was performed on 7500 fast Real-time PCR System (Applied Biosystems) for 40 cycles (15 seconds at 95°C, 15 seconds at 60°C and 30 seconds at 72°C) and then performed dissociation analysis (melt-curve) on the reactions to identify the characteristic peak associated with primer-dimers in order to separate from the single prominent peak representing the successful PCR amplification. We first determined the appropriate cycle threshold (Ct) using the automatic baseline determination feature. Fold variations in expression of an mRNA between RNA samples were calculated. Real time PCR primer sequences are sense: 5' GCCCTTCTTGTGCTCTTCGT3' and antisense: 5' GTTGGTGATGCCGTGCTT 3' for CACNA1C (mouse); sense: 5' CATCATCGGCAACATCCT 3' and antisense: 5' GAGGTCACAGGCTAAGTTC 3' for M3 (mouse); GADPH was served as an internal standard.
Western blot analysis
Mouse atrial tissue samples were directly frozen in liquid nitrogen following excision and further reduced to powder with a mortar. Powdered tissue was then resuspended in a cold (4 ºC) extraction buffer containing in mM: 10 Tris (pH 7.4), 250 sucrose and Complete EDTA-free Protease Inhibitor Cocktail (Roche, Applied Science, USA) to avoid protein degradation. The sample mixture was homogenized mechanically, incubated for 20 min on ice, and then centrifuged at 100×g for 10 min to remove debris and nuclei. The supernatant was then collected and the pellet was homogenized again. The latter procedure was repeated for three times to increase the efficiency of protein extraction. Collected supernatant was finally centrifuged at 100,000×g using an Optima LE-80K ultracentrifuge (Beckman, Instruments, CA, USA). The pellet corresponding to the membrane fraction was resuspended in 1% Triton-X100 cold extraction buffer and stored at -80 ºC. Membrane proteins (~50 µg) were fractionated by SDS polyacrylamide gel electrophoresis (7.5% polyacrylamide gels) and transferred to polyvinyl difluoride (PVDF) membranes. After transfer, membranes were blotted overnight with anti-M3 (1:1000) antibodies (Santa Cruz Biotechnology, , CA, USA) and goat polyclonal anti- CACNA1C (Santa Cruz Biotechnology, CA, USA), respectively. The next day, membranes were incubated for 2 h with the secondary antibody (goat anti-rabbit IgG, 1:10000. Bands were visualized with enhanced chemiluminescence. GAPDH was used as an internal control for equal input of protein samples, using anti-GAPDH antibody. Western blot bands were quantified using Quantity One software by measuring the band intensity (Area x OD) for each group and normalizing to GAPDH. The final results are expressed as fold changes by normalizing the data to the control values.
Data analysis and statistics
Clampfit 7.0 (Axon) and Original 7.0 were used for data analysis. Group data are expressed as mean ± S.D. Two-way analysis of variance (ANOVA) and Bonferroni-adjusted t-tests (in the case of significant inter-group differences by ANOVA) were used for statistical comparisons of current-voltage relations. Non-paired t-tests were applied for inter-group comparisons of AF duration and western blot analysis. P value less than 0.05 was considered to be significant.
Results
Intracardiac electrophysiology
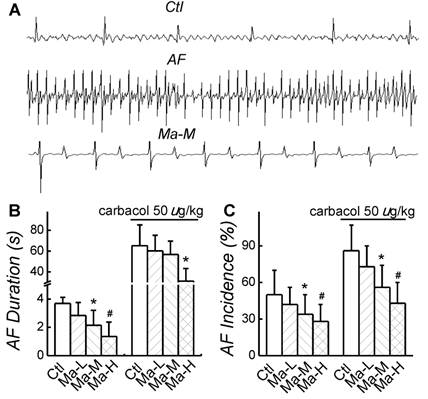
In control group, AF incidence induced by electric pacing was 50 ± 20% and AF duration was 3.68 ± 0.84 s; after application of carbachol 50 µg/kg, both AF incidence and AF duration were significantly increased (P < 0.05) up to 86 ± 21% and 65.2 ± 20.1 s, respectively. Compared with control group, application of Matrine decreased AF incidence and shortened AF duration in a dose-dependent manner. The similar results of Matrine were also confirmed in the presence of carbachol 50 µg/kg. Matrine 30 mg/kg decreased AF incidence to 34 ± 16% and shortened AF duration to 2.14 ± 1.06 s; after application of carbachol 50 µg/kg, AF incidence was 56 ± 18% and AF duration was 56.7 ± 13.3 s (n = 6, P < 0.05) (Fig. 2).
Effects of Matrine on AF duration and incidence. (A) Representative intracardiac recordings. (B) and (C) Effects of pretreatment of Matrine 30 mg/kg on AF duration and AF incidence after electrical pacing. *P < 0.05 and #P < 0.05 versus control.
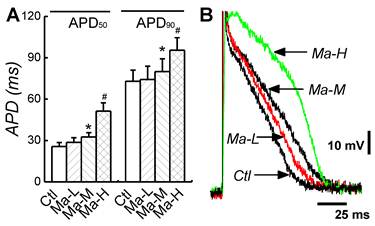
Effects of Matrine on APD of atrial myocytes. (A) Representative action potential traces recorded from single atrial cardiomyocytes of each group. (B) Comparisons of APD50 and APD90 of each group. *P < 0.05 and #P < 0.05 versus control.
Effects of Matrine on Action Potential Duration
To evaluate the effects of Matrine on the electric activity of atrial cardiomyocytes, the AP was recorded from isolated atrial cardiomyocytes; APD and ERP represented as the ratio of APD50/APD90 (RatioAPD50/90) were also quantified. In control group, APD50 and APD90 were 25.7 ± 2.81 ms and 72.9 ± 8.16 ms. Treatment of Matrine prolonged APD in a dose-dependent manner. In the case of Matrine 45 mg/kg, both APD50 and APD90 were significantly increased to 51.4 ± 5.89 ms and 95.5 ± 9.01 ms (n = 8, P < 0.05) (Fig. 3), respectively. Importantly, the RatioAPD50/90 was absolutely increased from 0.35 ± 0.07 up to 0.54 ± 0.06 (P < 0.05, n = 8) as shown in Table 1.
Comparison of action potential duration (APD) in each group.
| Control | Ma-L | Ma-M | Ma-H | |||||
|---|---|---|---|---|---|---|---|---|
| APD50 (ms) | 25.2±2.81 | 28.6±4.44 | 32.6±3.98* | 51.4±5.89# | ||||
| APD90(ms) | 72.9±8.16 | 74.2±9.61 | 80,3±9.19* | 95.5±9.01* | ||||
| APD50/APD90 | 0.35±0.07 | 0.38±0.03 | 0.39±0.03* | 0.54±0.06# |
APD50 50% repolarization of action potential duration; APD90 90% repolarization of action potential duration (n = 8, P* < 0.05; P#<0.01)
Atrial tachypacing effects on IKM3
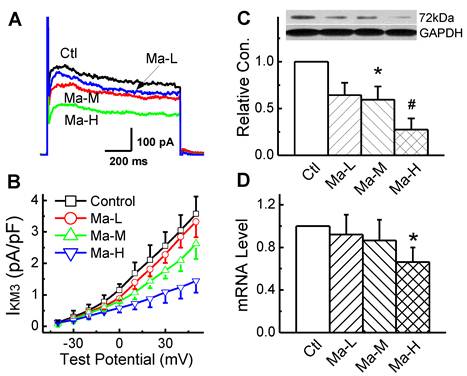
IKM3 was elicited by depolarizing voltage steps in the presence of 10 mM choline and 0.1 mM CdCl to block ICa-L in the bath solution and the step-current amplitude was measured at the end of 2 s step. Representative IKM3 recordings were illustrated in Fig. 4A. Matrine blocked the currents in a dose-dependent manner. At the test potential of +50 mV, the amplitude of IKM3 was 3.57 ± 0.55 pA/pF in control group and this IKM3 was significantly reduced to 2.62 ± 0.48 pA/pF and 1.44 ± 0.37 pA/pF (n = 8, P < 0.05) in the groups pretreated with Matrine 30 mg/kg and 45 mg/kg, respectively.
To determine whether the alterations of IKM3 density in atrial cardiomyocytes can be explained by alterations of the corresponding M3-R mRNA and protein expression levels. For the quantification of M3-R in both mRNA and protein levels, Real-time PCR and Western blot were performed with membrane samples extracted from atria, respectively. As shown in Fig. 4C and 4D, Matrine down-regulated the expression level of M3-R mRNA and protein dose-dependently.
Effects of Matrine on L-type calcium currents
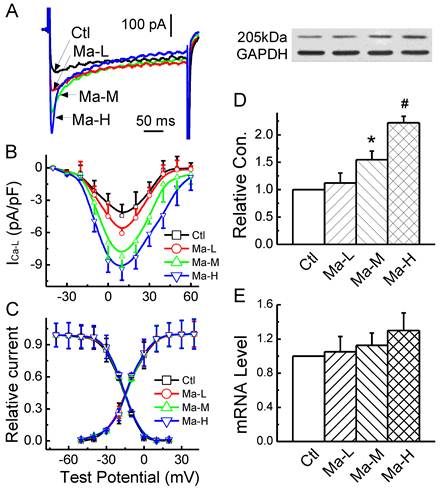
Based upon the effect of Matrine on APD, the changes in Ca2+ current mediated by Matrine would be expected. In the present experiment, the effect of Matrine on ICa-L was observed, current-voltage relationship (I-V curve) and voltage-dependent activation/inactivation profiles of ICa-L were analyzed. The results showed that ICa-L was increased by Matrine does-dependently (Fig. 5A and 5B) without changing the peak voltage (+10 mV). At the test potential of +10 mV, the current density was lower in cells from control group (-4.43 ± 1.02 pA/pF), compared with Matrine 30 mg/kg pretreatment group (-8.21 ± 1.22 pA/pF, n = 8, P < 0.05). In addition, Matrine had no effect on the voltage-dependent activation and inactivation profiles (Fig. 5C).
To further confirm the electrophysiological observation of Matrine on ICa-L, western blot analysis (Fig. 5D and 5E) and Real-time PCR were also performed. And the results indicated that Matrine only increased functional expression of the membrane protein α1C/Cav1.2, which is consistent with the change in current density of ICa-L. While as Matrine had not significant increase in mRNA level of α1C/Cav1.2.
Effects Matrine on M3-R expression and density of IKM3 in atrium. (A) Normalized traces of IKM3 elicited by 300 ms voltage step at test potential of +50 mV were derived from each group. (B) Current voltage (I-V) plots were shown for IKM3 of each group. (C) Representative image for M3-R expression of atrium from each group. Data were normalized to GAPDH. (D) The mRNA level of M3-R was analysed by real-time PCR. *P < 0.05 and #P < 0.01 versus control.
Effects Matrine on α1C/Cav1.2 expression and density of ICa-L in atrium. (A) Normalized traces of ICa-L elicited by 300 ms voltage step at +10 mV were derived from each group. (B) and (C) Current voltage (I-V) plots and voltage-dependent activation and inactivation profiles were shown for ICa-L of each group. (D) Representative image for α1C/Cav1.2 expression and GAPDH from each group. Data were normalized to GAPDH. (E) The mRNA level of α1C/Cav1.2 was analysed by real-time PCR. *P < 0.05 and #P < 0.01 versus control.
Discussion
The plausible anti-AF mechanisms of Matrine have been investigated in this study, which clearly demonstrates, at the first time, that Matrine can modulate M3-R and L-type Ca2+ channel to exert anti-AF effect. And this notion is strongly supported by our following evidence: (1) Matrine could decrease AF incidence and shorten AF duration in vivo study; (2) Matrine could up-regulate the expression of α1C/Cav1.2 and ICa-L and down-regulate the M3-R expression and IKM3; and (3) consequently Matrine absolutely prolongs the APD and ERP as well by increasing the ratio of ERP/APD.
Animal models of AF were developed using long term rapid atrial pacing in large animals, such as dog and goat [17, 31, 32]. In small animals such as the mouse, it has widely been accepted that the induction of fibrillatory tachycardia is impossible due to lack of a critical mass of the heart [33]. In our experiment, the intracellular pacing was performed by the first 6-second burst with a cycle length (CL) of 40 ms, decreasing in each successive burst with a 2-ms decrement down to a CL of 20 ms. Despite smaller mass of the normal mouse atria, it provides a micro-reentrant atrial in an intact normal mouse for initiating sustained reentrant circuit (Fig. 2A), which allow us investigating the anti-AF effects and possible mechanisms of Matrine in mouse atrium. The cholinergic discharge increases the susceptibility of AF through muscarinic receptor-mediated shortening of the atrial APD and ERP [34-36]. So Carbachol 50 µg/kg has been selected to decrease refractoriness in atrial tissue and thereby shorten the wavelength of cardiac cycles, and expected results have been confirmed in current study, which demonstrates that Carbachol markedly increases and prolongs the incidence and duration of AF, conceivably allows for the maintenance of atrial tachycardia circuits. Intriguingly, in Matrine groups, incidence and duration of AF induced by intracellular electric pacing significantly decreased and shortened, especially in 30 mg/kg Matrine group. These results strongly suggest the anti-AF effect of Matrine.
The molecular mechanisms of ion channel remodeling in AF, such as down-regulation of mRNA and protein functional expressions of Kv4.3, the α1c subunit of L-type Ca2+ channels, and the α-subunit of cardiac Na+ channels were observed through the dog chronic AF models and cultured atrial cardiomyocytes applied by electrical pacing [16,37,38]. So far, there are no reports about the ion channel remodeling in intact mice with AF. In the present study, we used programmed pacing to initiate AF and sustain AF for certain time period by choosing the best cycle length of intracellular electric pacing in order to detect the membrane expression of M3-R, Cav1.2 channel, and other ionic currents.
It is now well recognized that rapid pacing causes electrical remodeling in the atrium that aggravates AF. With rapid pacing, the onset of remodeling is rapid with shortening of atrium repolarization in minutes or hours. Calcium overload via the ICa-L in AF is thought to play a key role in ion channel remodeling and apoptosis. Under this circumstance, cardiomyocytes might trigger cytoprotective mechanisms by down-regulation of mRNA and proteins encoded with L-type Ca2+ channels, and acceleration of its inactivation to reduce the Ca2+ influx. In clinical management of patients with AF, calcium channel blocker such as Verapamil is usually used to maintain sinus rhythm and decrease calcium overload. However, it may promote AF by shortening the atrial APD and ERP. In our experiment, we found that Matrine not only significantly shortened the duration of AF but also dramatically increased the chances of spontaneous termination of AF. These electrophysiological data were consistent with our molecular evidence that Matrine up-regulated α1c subunit protein expression without effect on mRNA level and increased the current density of ICa-L contributing to the prolongation of APD and ERP. In addition, Matrine could also prolong APD by inhibiting K+ channels [28], consequently prolonged a repolarization and further increased the ERP of the myocardium. Even though Matrine increases Ca2+ influx by activating Ca2+ channel, it does not induce Ca2+ overload through other cardiac protective mechanisms, such as inhibited an apoptosis of myocardium and inhibited calcium overload induced by Ang II [39].
Both M2 and M3 muscarinic receptor-mediated K+ currents may be involved in the development of AF. In heart, M2-R-regulated IKACh comprises >60% of the total muscarinic mediated outward K+ current. However, with AF due to ventricular tachypacing-induced congestive heart failure, the density of M2 receptors was significantly down-regulated with the reduction of IKACh current density in atrial cells. In sharp contrast, M3-R expression and IKM3 current density was remarkably increased [12]. Intriguingly, our data demonstrated that the M3-R expression and IKM3 current densities were both reduced by the pretreatment with Matrine compared with normal atrial cardiomyocytes, resulting in the prolongation of APD/ERP and termination of re-entry. Pathological conditions may be another factor influencing the expression and relative contribution of the cardiac M3-R, in other words, a minor role of the M3-R under physiological conditions might become prominent under pathological situations [12]. So M3-R may be a new target for treatment of AF.
Electrical remodeling due to rapid atrial rates has a central role in pathogenesis of AF. Decrease in IL-Ca, increase in inward rectifier K+ current (IK1), and active M3-R activated K+ current (IKM3) are all contribute to AF by APD shortening and functional re-entry. Traditional anti-AF drugs simply inhibit K+ currents (class III) or Ca2+ currents (class Ⅳ). More importantly, Class III drugs suppress re-entry by increasing refractory periods, but can produce torsade de pointes arrhythmias that are related to early after-depolarizations. Class IV agents are frequently used to reduce Ca2+ overload, but it aggravates down-regulation of ICa-L in the atrium and promotes the risk of ventricular arrhythmia without the therapeutic effect on AF. However, these data demonstrated that Matrine possessed anti-AF effects through multiple targets, such as, the inhibition of M3-R mediated IKM3 and up-regulation of ICa-L.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (No 30973532) and Returned Oversea Scholars' Foundation of Heilongjiang Province (No LC2011C04) and Ph.D. Programs Foundation of Ministry of Education of China (No 20112307120010).
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Dobrev D, Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet. 2010;375:1212-3
2. Fuster V, Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL & Wann S. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation - executive summary: a report of the American College of Cardiology/American Heart Association Task Force and European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation). Eur Heart J. 2006;27:1979-2030
3. Pedersen OD, Abildstrom SZ, Ottesen MM, Rask-Madsen C, Bagger H, Køber L & Torp-Pedersen C. TRACE Study Investigators: Increased risk of sudden and non-sudden cardiovascular death in patients with atrial fibrillation flutter following acute myocardial infarction. Eur Heart J. 2006;27:290-5
4. Tsang TS, Miyasaka Y, Barnes ME & Gersh BJ. Epidemiological profile of atrial fibrillation: a contemporary perspective. Prog Cardiovasc Dis. 2005;48:1-8
5. Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nat Rev Drug Discov. 2006;5:1034-49
6. Nattel S, Opie LH. Controversies in atrial fbrillation. Lancet. 2006;367:262-72
7. Schauerte P, Scherlag BJ, Pitha J, Scherlag MA, Reynolds D, Lazzara R & Jackman WM. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. 2000;102:2774-80
8. Zang WJ, Yu XJ, Honjo H, Kirby MS & Boyett MR. On the role of G protein activation and phosphorylation in desensitization to acetylcholine in guinea-pig atrial cells. J Physiol. 1993;464:649-79
9. Shi H, Wang H, Lu Y, Yang B & Wang Z. Choline modulates cardiac membrane repolarization by activating an M3 muscarinic receptor and its coupled Kt channel. J Membr Biol. 1999;169:55-64
10. Shi H, Wang H & Wang Z. Identification and characterization of multiple subtypes of muscarinic acetylcholine receptors and their physiological functions in canine hearts. Mol Pharmacol. 1999;55:497-507
11. Wang H, Shi H, Lu Y, Yang B & Wang Z. Pilocarpine modulates the cellular electrical properties of mammalian hearts by activating a cardiac M3 receptor and a K+ current. Br J Pharmacol. 1999;126:1725-34
12. Shi H, Wang H, Li D, Nattel S & Wang Z. Differential alterations of receptor densities of three muscarinic acetylcholine receptor subtypes and current densities of the corresponding Kt channels in canine atria with atrial fibrillation induced by experimental congestive heart failure. Cell Physiol Biochem. 2004;14:31-40
13. Tuomi JM, Chidiac P & Jones DL. Evidence for enhanced M3 muscarinic receptor function and sensitivity to atrial arrhythmia in the RGS2-deficient mouse. Am J Physiol Heart Circ Physiol. 2010;298:H554-61
14. Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Müller FU, Schmitz W, Schotten U, Anderson ME, Valderrábano M, Dobrev D & Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940-51
15. Mancarella S, Yue Y, Karnabi E, Qu Y, El-Sherif N & Boutjdir M. Impaired Ca2+ homeostasis is associated with atrial fibrillation in the alpha1D L-type Ca2+ channel KO mouse. Am J Physiol Heart Circ Physiol. 2008;295:H2017-24
16. Van der Velden HMW, van der Zee L, Wijffels MC, van Leuven C, Dorland R, Vos MA, Jongsma HJ & Allessie MA. Atrial fibrillation in the goat induces changes in monophasic action potential and mRNA expression of ion channels involved in repolarization. J Cardiovasc Electrophysiol. 2000;11:1262-69
17. Yue L, Melnyk P, Gaspo R, Wang Z & Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ Res. 1999;84:776-84
18. Fareh S, Bénardeau A & Nattel S. Differential efficacy of L- and T-type calcium channel blockers in preventing tachycardia-induced atrial remodeling in dogs. Cardiovasc Res. 2001;49:762-70
19. Bénardeau A, Fareh S & Nattel S. Effects of verapamil on atrial fibrillation and its electrophysiological determinants in dogs. Cardiovasc Res. 2001;50:85-96
20. Zhu XH, Qiu YD, Shi MK, Wu B, Zheng XG & Ding YT. Effect of matrine on cold ischemia and reperfusion injury of sinusoidal endothelial cells in rat orthotop ic liver transp lantation. Acta Pharm Acol Sin. 2003;24:169-74
21. Zhang MJ, Huang J. Recent research progress of anti-tumor mechnism matrine. Zhongguo Zhong Yao Za Zhi. 2004;29:115-8
22. Long Y, L in XT, Zeng KL& Zhang L. Efficacy of intramuscular matrine in the treatment of chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2004;3:69-72
23. Lao Y. Clinical study of matrine injection on p reventing liver function damage of anti-tumor drugs during chemo therapy of breast cancer. J Chin Med Mater. 2005;28:735-7
24. Li X, Chu W, Liu J, Xue X, Lu Y, Shan H & Yang B. Antiarrhythmic properties of long-term treatment with matrine in arrhythmic rat induced by coronary ligation. Biol Pharm Bull. 2009;32:1521-6
25. Zhang Y, Zou YN, Hao DX, L in HX & Xiao JN. Therapeutic effect of amiodarone compared with matrine and p rocainamide on ventricular arrhythmias in coronary heart disease. J Harbin Med Univ. 2005;39:344-8
26. Zhou YH, Xu CX, Shan HL, Lu YJ & Yang BF. Inhibition of matrine on potassium currents in guinea pig ventricular myocytes. Chinese J Pharmacol and Toxicol. 2007;21:167-73
27. Zhou YH, Shan HL, Qiao GF, Sui XH, Lu YJ & Yang BF. Inotropic Effects and Mechanisms of Matrine, a main alkaloid from Sophora flavescens Ait. Biol Pharm Bull. 2008;31:2057-62
28. Ling JY, Zhang GY, Cui ZJ & Zhang CK. Supercritical fluid extraction of quinolizidine alkaloids from Sophora flavescens Ait. and purification by high-speed counter-current chromatography. J Chromatogr A. 2007;23:123-7
29. Berul CI, Aronovitz MJ, Wang PJ & Mendelsohn ME. In vivo cardiac electrophysiology studies in the mouse. Circulation. 1996;94:2641-8
30. Wakimoto H, Maguire CT, Kovoor P, Hammer PE, Gehrmann J, Triedman JK & Berul CI. Induction of atrial tachycardia and fibrillation in the mouse heart. Cardiovasc Res. 2001;50:463-73
31. Morillo CA, Klein GJ, Jones DL & Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588-95
32. Ausma J, Wijffels M, Thoné F, Wouters L, Allessie M & Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation the goat. Circulation isolated canine right atrium. Circ Res. 1992;71:1254-67
33. Garrey WE. The nature of fibrillary contraction of the heart: Its relation to tissue mass and form. Am J Physiol. 1994;33:397-414
34. Schuessler RB, Rosenshtraukh LV, Boineau JP, Bromberg BI & Cox JL. Spontaneous itachyarrhythmias after cholinergic suppression in the isolated perfused canine right atrium. Circ Res. 1991;69:1075-87
35. Schuessler RB, Grayson TM, Bromberg BI, Cox JL & Boineau JP. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circ Res. 1992;71:1254-67
36. Rosenshtraukh LV, Zaitsev AV, Fast VG, Pertsov AM & Krinsky VI. Vagally induced block and delayed conduction as a mechanism for circus movement tachycardia in frog atria. Circ Res. 1989;64:213-26
37. Comtois P, Sakabe M, Vigmond EJ, Munoz M, Texier A, Shiroshita-Takeshita A & Nattel S. ACC/AHA/ESC 2006 guidelines for the rapidly unbinding Na+ channel blockers: insights from mathematical models and experimental correlates. Am J Physiol Heart Circ Physiol. 2006;295:H1489-504
38. Cha TJ, Ehrlich JR, Chartier D, Qi XY, Xiao L & Nattel S. Kir3-based inward rectifier potassium current. Potential role in atrial tachycardia remodeling effects on atrial repolarization and arrhythmias. Circulation. 2006;113:1730-7
39. Yang L, Wang BH, Zhou CH & Bi YY. Matrine induces apoptosis in angiotensin II-stimulated hyperplasia of cardiac fibroblasts: effects on Bcl-2/Bax expression and caspase-3 activation. Basic Clin Pharmacol Toxicol. 2007;101:1-8
Author Biography
Dr Yuhong Zhou works for the Department of Pharmacology, Harbin Medical University, which focuses on understanding the mechanism of cardiovascular drug. The Department is the State-Province Key Laboratories of Biomedicine-Pharmaceutics of China and one of the Key Laboratory of Cardiovascular Research affiliated to Ministry of Education in China. Dr Zhou is an associated professor and research scientist with awards for outstanding and innovative research in the molecular and electrophysiological mechanisms of Chinese traditional herbs.
Dr Guofen Qiao is a research scientist with 20 years of research experience in the cardiovascular Pharmacology. Dr. Qiao's research has been fully funded by the funding agencies such as the National Nature & Science Foundation, Provincial Key Research Foundation, and Provincial Educational Committee and highly honoured for several times by central and local department of science and technology. She has published more than 45 research papers internationally.
![]() Corresponding author: Dr. Qiao Guofen, Department of Pharmacology, Harbin Medical University, Bao-Jian Road 157, Harbin 150081, China. Email: qiaogf88com.cn
Corresponding author: Dr. Qiao Guofen, Department of Pharmacology, Harbin Medical University, Bao-Jian Road 157, Harbin 150081, China. Email: qiaogf88com.cn
Received 2011-8-9
Accepted 2011-12-4
Published 2011-12-9
