ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2023; 19(14):4552-4570. doi:10.7150/ijbs.80323 This issue Cite
Research Paper
Protein Arginine Methyltransferases Refine the Classification of Clear Cell Renal Cell Carcinoma with Distinct Prognosis and Tumor Microenvironment Characteristics
1. Department of Urology, Fudan University Shanghai Cancer Center, Shanghai 200032, P.R. China.
2. Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, P.R. China.
3. Shanghai Genitourinary Cancer Institute, Shanghai 200032, P.R. China.
4. Department of Neurosurgery, Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, 533000, P.R. China.
5. Department of Interventional Oncology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China.
6. The Affiliated Jiangsu Shengze Hospital of Nanjing Medical University, Suzhou 215228, P.R. China.
# These authors contributed equally to this work.
Abstract
Background: Clear cell renal cell carcinoma (ccRCC) is an aggressive urological cancer that originates from the proximal tubular epithelium. As one of the most common post-translational modification, protein arginine methylation plays a pivotal role in various cancer-associated biological functions, especially in cancer immunity. Therefore, constructing a protein arginine methylation-related prognostic signature would be beneficial in guiding better personalized clinical management for patients with ccRCC.
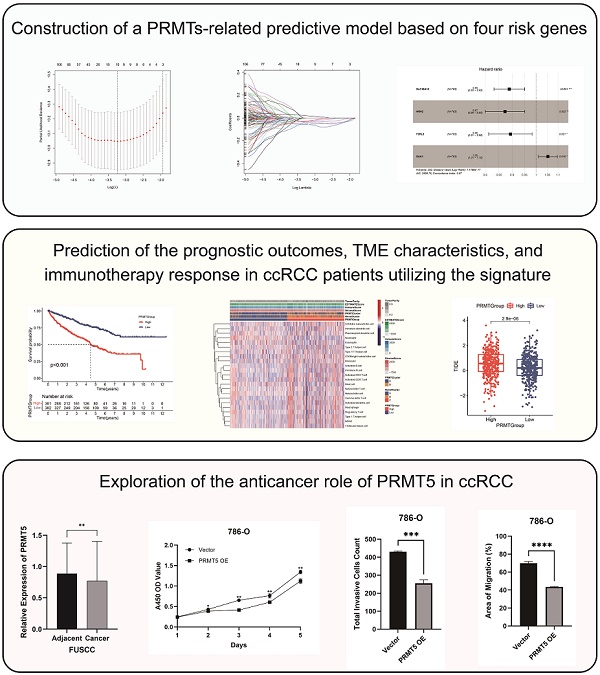
Methods: Based on the multi-omics profiling of the expression levels of eight protein arginine methyltransferases (PRMTs) in 763 ccRCC samples (from TCGA, CPTAC, EMBL, and ICGC databases), we established a scoring system with machine-learning algorithms to quantify the modification patterns on clinical and immunological characterizations of individual ccRCC patient, which was termed as PRMTScore. Moreover, we utilized two external clinical cohorts receiving immunotherapy (n=302) to validate the reliability of the PRMTScore system. Multiplex immunohistochemistry (mIHC) was performed to characterize the cellular composition of 30 paired ccRCC samples. The proteomic profiling of 232 ccRCC samples obtained from Fudan University Shanghai Cancer Center (FUSCC) was analyzed to validate the protein expression of PRMT5 in ccRCC. Finally, CCK-8, transwell, and wound healing assays were conducted to elucidate the role of PRMT5 in ccRCC in vitro.
Results: A total of 763 ccRCC patients with available multi-omics profiling were stratified into two clusters (PRMTCluster A and B) with distinctive prognosis, genomic alterations, tumor microenvironment (TME) characteristics, and fundamental biological mechanisms. Subsequently, protein arginine methylation-related prognostic signature (PRMTScore) was constructed and consisted of SLC16A12, HRH2, F2RL3, and SAA1. The PRMTScore showed remarkable differences in outcomes, immune and stromal fractions, expressions of immune checkpoints, the abundance of immune cells, and immunotherapy response in ccRCC patients. Additionally, preliminary insights unveiled the tumor-suppressive role of PRMT5 in ccRCC, and the signal of PRMT5low significantly predicted aggressive prognosis and the high abundance of PD1+ CD8+ cells in ccRCC.
Conclusion: We constructed a PRMTScore system, which showed the potent ability to assess the prognosis, TME characteristics, and immunotherapy response for patients with ccRCC. Moreover, this is the first study to propose that PRMT5 acts as a cancer suppressor in ccRCC.
Keywords: clear cell renal cell carcinoma, protein arginine methylation, prognosis, tumor microenvironment, immunotherapy response, PRMT5
