ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2023; 19(14):4588-4607. doi:10.7150/ijbs.86911 This issue Cite
Review
Reversing Gray Hair: Inspiring the Development of New Therapies Through Research on Hair Pigmentation and Repigmentation Progress
1. Department of Dermatology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, China.
2. Department of Dermatology, Xuzhou Medical University, Xuzhou, China.
# These authors have contributed equally to this work.
Abstract
Hair graying is a common and visible sign of aging resulting from decreased or absence of melanogenesis. Although it has been established that gray hair greatly impacts people's mental health and social life, there is no effective countermeasure other than hair dyes. It has long been thought that reversal of gray hair on a large scale is rare. However, a recent study reported that individual gray hair darkening is a common phenomenon, suggesting the possibility of large-scale reversal of gray hair. In this article, we summarize the regulation mechanism of melanogenesis and review existing cases of hair repigmentation caused by several factors, including monoclonal antibodies drugs, tyrosine kinase inhibitors (TKIs), immunomodulators, other drugs, micro-injury, and tumors, and speculate on the mechanisms behind them. This review offers some insights for further research into the modulation of melanogenesis and presents a novel perspective on the development of clinical therapies, with emphasis on topical treatments.
Keywords: hair repigmentation, hair pigmentation, melanogenesis, targeting drugs, topical treatments
Introduction
Hair graying is a common, visible, and early marker of human aging [1, 2]. It is now understood that human hair and its pigmentation can greatly affect societal perception, emotional well-being, and psychological state [3]. Considering the potential health risks posed by hair dyes [4], new strategies for hair color change are warranted.
Hair graying has long been thought of as an irreversible age-related process. Nonetheless, recent research has revealed that restoring the color of a single gray hair to its original pigmentation is a general phenomenon regardless of age, gender, ethnicity, and corporeal regions but only appears in a single anagen of rare HFs [5]. Although there is great heterogeneity between hair follicles (HF), the similarities between the processes of graying and repigmentation imply the potential for systemic behavioral factors (such as life stress) to simultaneously regulate the pigmentation of multiple HFs. Meanwhile, proteomics and computational simulation have proven the theoretical possibility of reversing gray hair temporarily [5]. Based on the evidence presented, it becomes evident that the prevention or reversal of hair graying holds significant promise for the future. Therefore, in this review, we summarize the regulation of melanogenesis and focus on cases of hair repigmentation and the mechanisms behind them, trying to inspire future research on the regulation of melanogenesis and therapy development.
Overview of hair pigmentation and hair graying
Melanocytes in human HF are classified into several sub-populations according to function, differentiation status, and location. Within anagen hair follicles, melanocytes responsible for hair pigmentation primarily reside in the hair matrix surrounding the mid to upper dermal papilla. These bulbar melanocytes express active tyrosinase and the melanogenic intermediate dihydroxyphenylalanine (DOPA) and are considered a component of hair follicle pigment unit (HFPU) [1, 6]. Melanogenesis occurs in specialized lysosomal-related organelles termed melanosomes. The melanin-containing melanosomes are then transferred to the keratinocytes of hair shaft through dendritic and filopodial processes [7]. Melanocyte stem cells (McSCs) are located in the bulge and the sub-bulge area of the outer root sheath (ORS). These cells are immature and poorly or un-pigmented [6]. Recent studies have indicated that the majority of melanocyte stem cells possess a unique and unexpected mechanism for self-renewal and melanogenic melanocyte production. These McSCs exhibit a distinctive ability to switch between transit-amplifying and stem cell stages, which fundamentally distinguishes them from other self-renewing systems [8]. According to live imaging and single-cell RNA sequencing, McSCs move between the transit-amplifying and hair follicle stem cell compartments through dedifferentiation, reversibly entering multiple differentiation stages controlled by the local microenvironment. Long-term lineage tracing studies have provided compelling evidence that the sustained melanocyte stem cell system is supported by reverted McSCs that dedifferentiate from transit-amplifying stage rather than reserved population of stem cells that inherently maintained in an undifferentiated state.
Indeed, hair pigmentation and the hair cycle are inextricably linked. The hair cycle consists of three distinct stages: anagen, catagen, and telogen. Hair pigmentation only happens during anagen because the melanogenic HFPU exists in this period [9-11]. Most differentiated melanocytes experience apoptosis in the catagen phase, while bulge McSCs survive in the secondary hair germ [12-14]. As a new anagen phase is initiated, the surviving McSCs differentiate into melanogenic melanocytes to rebuild the HFPU [13, 15].
Current evidence suggests that multiple factors can influence the process of hair graying [6]. However, the root cause of hair graying is the dysfunction and cell death of melanogenic melanocytes in the HFPU. It is widely thought that during a single anagen phase, the HFPU is self-maintained and does not require replenishment from McSCs [1]. Therefore, the initial onset of hair graying is not necessarily related to the depletion of McSCs. However, as individuals age, stranded McSCs accumulate over time and do not contribute to the production of mature melanocytes [8, 16, 17]. The preservation of McSCs is crucial for the reconstruction of the HFPU and provides the possibility for the reversal of grey hair. Once McSCs are exhausted, hair graying becomes irreversible [1].
Epithelial stem cells (EpSCs) in the hair follicle are crucial in providing a functional niche for melanocyte stem cells [18, 19]. The offspring of EpSCs in the HF bulge and hair germ develop into outer root sheath (ORS) and transit-amplifying cells (TACs) in the HF matrix, which support HF regeneration. The TACs differentiate into several lineages that eventually give rise to the hair shaft and its supporting components [20]. Recently, single-cell transcriptomics revealed that P53 pathway activation-induced specific depletion of matrix TAC, but not HFSCs, is associated with early-stage human hair graying [20]. Therefore, the effects of regulatory factors on cells within the HF, other than melanocytes, are also considered in this context.
It is crucial to note that even visually colorless scalp HFs may still have a few hair bulb melanocytes. Some may even continue to produce melanin, although they lack dendritic morphology and melanin transmission to the hair shaft [21]. Thus, it is theoretically possible that a special therapy that reverses hair graying before all the hair bulb melanocytes and McSCs disappear can be developed in the future.
Signaling pathways in the regulation of melanogenesis
Wnt/β-catenin signaling pathway
When Wnt molecules bind to their receptors, β-catenin is activated, increasing Melanocyte Inducing Transcription Factor (MITF) transcription in McSCs. Glycogen synthase kinase 3β (GSK3β) phosphorylates β-catenin without Wnt signaling, which causes it to break down through a proteasome-dependent mechanism. The activation of Wnt signaling also increases Endothelin Receptor Type B (EDNRB) signaling [22, 23]. These effects of Wnt signaling synergistically promote MsSCs' migration, proliferation, differentiation, and melanogenesis. Moreover, simultaneous activation of Wnt/β-catenin signaling in EpSCs and McSCs initiates pigmented hair regeneration [24].
MC1R
As one of the central regulators of pigmentation, Melanocortin 1 Receptor (MC1R) signaling promotes melanogenesis and melanosome transfer in melanocytes. Interestingly, it has been shown that ultraviolet B (UVB) induces keratinocytes to express pro-opiomelanocortin (POMC), which is cleaved to release α-melanocyte-stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH), both ligands of MC1R [25]. MC1R activates adenylyl cyclase and then increases cyclic adenosine monophosphate(cAMP). One of the numerous effects of cAMP, mediated by cAMP-dependent protein kinase A (PKA), is the phosphorylation of cAMP-responsive element-binding protein (CREB), which stimulates MITF transcription [26].
Store‐operated Ca2+ entry (SOCE) is initiated upon endoplasmic reticulum (ER) Ca2+ release. The Ca2+ binding ER membrane protein stromal interaction molecule 1 (STIM1) senses the Ca2+ store depletion and then oligomerizes and interacts with plasma membrane store-operated Ca2+ (SOC) channel Orai1, leading to Ca2+ influx [27]. α-MSH-induced cAMP stimulates ER Ca2+ release through phospholipase C (PLC)/ inositol triphosphate (IP3) signaling. Subsequently, STIM1 activates the plasma membrane-localized adenylyl cyclase 6 (ADCY6), which acts independently of Orai1, to increase cAMP. This positive feedback loop controlled by cAMP‐Ca2+ crosstalk further promotes the effects of α-MSH [28].
In addition to promoting melanogenesis in melanocytes, the activation of MC1R also enhances the repair of Ultraviolet radiation (UVR)-induced DNA damage [29]. Besides, it has been reported that injury or UVB-induced epidermal migration of McSCs also relies on MC1R signaling [30].
SCF/C-KIT
The stem cell factor (SCF)/tyrosine kinase receptor (KIT) signaling system plays a significant role in melanogenesis. When SCF binds to its receptor c-KIT, it triggers the receptor's tyrosine kinase activity, which results in receptor phosphorylation. As a result of the phosphorylation of c-KIT, mitogen-activated protein kinase (MAPK) is stimulated and then triggers the phosphorylation of CREB, activating MITF [31]. An extracellular signal-regulated kinase (ERK) can be activated by c-KIT signaling. While ERK signaling activates CREB to promote melanogenesis, it has also been shown to phosphorylate MITF, leading to its ubiquitination and subsequent degradation, thus forming a feedback loop in melanin regulation [32]. However, it cannot be ignored that the presence of MITF alone is not sufficient for Tyr expression and that KIT signaling is needed not only for the proliferation and survival of melanoblasts but also for Tyr induction and the transition of melanoblasts to mature melanocytes [33].
EDN/EDNRB
Endothelin receptor B (EDNRB) has been identified as playing indispensable roles in the maintenance, proliferation, differentiation, and migration of McSCs [22]. Besides, EDNRB signaling activates PLC/IP3/ signaling and ER Ca2+ release [27]. Following SOCE, MITF expression significantly increases via the Ca2+/PKC/MAPK/p90 ribosomal S6 kinase (RSK) pathway, the Mitogen- and stress-activated kinase 1 (MSK1)/CREB pathway, and the PKA/CREB pathway [34-36]. The expression of EDNRB is regulated by MITF, suggesting EDNRB signaling and MITF expression form a self-reinforcing positive feedback loop that promotes melanocyte proliferation and melanogenesis [25]. After wounding or UVR, Endothelin 1 (EDN1)/EDNRB signaling enhances the proliferation and differentiation of McSC and increases the regeneration of epidermal melanocytes [22, 35].
PI3K/AKT
It is well-established that the phosphoinositide 3-kinase (PI3K) signaling pathway activates the serine/threonine-specific protein kinase (AKT) to increase GSK3β enzyme activity and prevent melanogenesis [37]. MC1R can activate PI3K/AKT signaling to produce a negative feedback effect on melanogenesis and stimulate the extracellular release of melanin, preventing oxidative stress, DNA damage, and reduced survival [38]. Although SCF/c-KIT is an upstream signal for PI3K/AKT activation in melanocyte and melanoma cells [39, 40], whether c-KIT has such a negative feedback loop remains to be demonstrated experimentally.
TGF-β
Transforming growth factor-β (TGF-β) is the major regulator of McSCs maintenance, causing cell cycle arrest, downregulation of MITF and melanogenic genes, which ultimately keeps McSCs in a state of immaturity and quiescence [41]. MITF and its downstream genes are the main targets of TGF-β in inhibiting melanogenesis [42-45]. The TGF-β/Smads pathway negatively regulates the paired-box homeotic gene 3 (PAX3), which works synergistically with the SRY-Box Transcription Factor 10 (SOX10) to upregulate MITF, dependent on a cAMP-response element (CRE) [46, 47]. Both TGF-β1 and TGF-β3 also downregulate MITF by activating ERK signaling to decrease melanogenesis [48, 49]. Lastly, but importantly, TGF-β1 and TGF-β2 are recognized as key catagen-inducing growth factors of HF [50, 51]. It has been demonstrated in melanoma cell lines that the direct transcriptional target of TGF-β1-the Kruppel-like transcription factor GLI2 not only suppresses MITF through PKA/cAMP signaling [52] but also directly downregulates tyrosinase-related protein 2 (TRP2) by competitive inhibition of CREB [53].
MITF
MITF is a critical transcriptional regulator of melanogenesis and McSC maintenance and differentiation [26, 54]. MITF positively regulates pigmentation-associated genes to promote differentiation-associated function and directly transactivates promoters of three primary melanogenesis enzymes, tyrosinase (TYR), tyrosine-related protein-1 (TYRP-1), and dopachrome tautomerase, also known as tyrosine-related protein-2, TYRP-2 (-2) [55]. MITF also upregulates genes contributing to melanosome function, such as G Protein-Coupled Receptor 143 (GPR143), SILV, and melanoma-associated antigens recognized by T cells (MART-1) or melanosome transport such as Rab27 and Myosin5a (MYO5a) [55, 56].
Furthermore, MITF plays a role in melanocyte proliferation. The capacity of MITF to increase cyclin-dependent kinase 2 (CDK2) expression highlights its functions as a pro-proliferative factor [57]. MITF also has been known to activate the transcription of T-box transcription factor 2 (TBX2) [58], which inhibits senescence by repression of p21 and p19 and participates in melanocyte growth and invasion [59, 60]. Furthermore, MITF directly upregulates cell cyclin-related genes cyclin B1 (CCNB1) and cyclin D1 (CCND1) and mitotic genes such as polo-like kinase 1 (PLK1) [61].
Several downstream genes of MITF promote cell survival. MITF is the positive regulator of anti-apoptotic factors B-cell-lymphoma 2 (BCL2) and baculoviral IAP repeat containing 7 (BIRC7) [62, 63] and is involved in the regulation of the oncogenic hepatocyte growth factor receptor MET and the type III ribonuclease DICER, a necessary regulator of microRNA processing, thereby performing its anti-apoptotic effects [64, 65]. It is widely thought that MITF mitigates DNA damage by increasing a group of repair genes, including DNA ligase I (LIG1), telomerase reverse transcriptase (TERT), essential meiotic endonuclease 1 homolog 1 (EME1), Breast Cancer 1 protein (BRCA1), and Fanconi anemia protein A (FANCA) [66]. MITF transcriptionally regulates General transcription factor IIH subunit 1 (GTF2H1), which encodes the core component of Transcription Factor IIH (TFIIH), and CDK7, which encodes TFIIH kinase to promote the rapid recovery of nucleotide excision repair [67]. In response to reactive oxygen species (ROS), MITF positively regulates apurinic-apyrimidinic endonuclease 1 (APE1), hypoxia-inducible factor 1 (HIF1α), and Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) to improve survival capacity under oxidative stress [68-70].
It has recently been revealed that MITF enhances STIM1 expression transcriptionally [71], indicating the presence of a positive feedback loop between STIM1 and MITF to promote the MITF-inducing effect of MC1R or EDNRB signaling.
MITF can be regarded as a final common pathway in modulating the biological behavior of McSC and melanocyte to some extent, as several external and internal factors converge on it through different intracellular signaling pathways, and their effects depend on downstream genes (Fig. 1).
Novel mechanisms of hair pigmentation regulation
Sympathetic nerves and sensory nerves
It is widely acknowledged that HFs are innervated by sympathetic and sensory nerves [72]. Under stressful conditions, these sympathetic nerves are hyperactivated and release noradrenaline in bursts, resulting in rapid McSC proliferation possibly mediated by β2 adrenergic receptors/AC/cAMP/PKA pathway. It has been shown that these sensory nerves can provide Sonic Hedgehog (SHH) signaling [73], which is required for the normal proliferation of melanocytes [74]. Then these McSC differentiate ectopically and migrate out of the hair bulge, eventually causing the permanent depletion of McSC and irreversible grey hair [75].
Neurotransmitters
Multiple neuropeptides released from intra-epidermal sensory nerves, such as calcitonin gene-related peptide (CGRP), substance P (SP), and vasoactive intestinal peptide (VIP), have been reported to regulate melanogenesis by acting on melanocytes directly or indirectly through immune and inflammatory responses [76, 77]. In this context, we will primarily focus on their direct effects on HFs and melanocytes.
CGRP exerts catagen-inducing effects on human HFs and can maintain and restore hair follicle immune privilege (IP) via repression of MHC class I antigen [78, 79]. Melanogenesis and melanocyte dendricity is enhanced by certain CGRP-induced keratinocyte-derived melanotrophic factors ex vivo [80]. In mice, CGRP from sensory neurons increases insulin-like growth factor-I (IGF-I) production in the HF dermal papilla cells, thereby promoting hair growth and melanogenesis [81]. However, not all studies support the conclusion that CGRP promotes melanin production. In this regard, it has been shown that in B16F10 cells, CGRP cooperates with SP to inhibit melanogenesis and induce cell apoptosis [82].
Signaling pathways in the regulation of melanogenesis. Melanogenesis and melanocytes proliferation, differentiation and survival are regulated by the MITF transcription factor, which is regulated by a number of important signaling pathways, including Wnt/β-catenin, KIT/SCF, ET-1/EDNRB, α-MSH/MC1R and TGF-β pathways. MITF: Melanocyte Inducing Transcription Factor; GSK3β: Glycogen synthase kinase 3β; AKT: serine/threonine-specific protein kinase; MC1R: Melanocortin 1 Receptor; TGF-β: Transforming growth factor-β; PI3K: phosphoinositide 3-kinase; SCF: stem cell factor; c-KIT: tyrosine kinase receptor; MAPK: mitogen-activated protein kinase; ERK: Extracellular signal-regulated kinase; ET-1: Endothelin 1; EDNRB: Endothelin receptor B; PKC: Protein kinase C; α-MSH: α-melanocyte-stimulating hormone; cAMP: cyclic adenosine monophosphate; PKA: cAMP-dependent protein kinase A; CREB: cAMP response element-binding protein; ACTH: adrenocorticotropic hormone; AC: adenylate cyclase; PAX3: paired-box homeotic gene 3; SOX10: SRY-Box Transcription Factor 10; CRE: cAMP-response element.

SP is released from sensory nerve endings induced by psychoemotional stress and regulates immune cells or HF mainly through neurokinin-1 receptors (NK1R) [83, 84]. In organ-cultured HFs, SP upregulates nerve growth factor (NGF) and its apoptosis- and catagen-inducing receptor (p75NTR), while it downregulates the growth-inducing NGF receptor neurotrophic tyrosine kinase receptor type 1 (TrkA). Furthermore, MHC class I and β2-microglobulin are upregulated, suggesting that SP impairs immune privilege (IP) [84]. Animal models have also shown that stress-induced SP not only inhibits HF keratinocyte proliferation and induces apoptosis but also promotes mast cell degranulation, causing the production of ROS and neurogenic inflammation, and then inhibits hair growth and induces the catagen phase in the hair cycle [83, 85, 86].
Studies have shown that in B10F16 melanoma cells, SP not only exerts a synergistic effect with CGRP, but also induces apoptosis in B10F16 cells and downregulates 5-hydroxytryptamine (5-HT)1A receptor and 5-HT2A receptor, both of which promote melanogenesis. This effect is mediated by binding to the NK1R receptor and activating S6 kinase 1 (S6K1) while inhibiting the MAPK signaling pathway [87-89]. Moreover, excess SP induced by mental stress decreases melanogenesis through keratinocytes. During this process, the hypothalamic-pituitary-adrenocortical (HPA) axis is key in mediating the effects of SP signaling [90]. However, contrasting studies revealed that SP promotes EDN1 secretion via endothelin-converting enzyme 1 and upregulates Wnt/ β-catenin signaling by downregulating the Wnt inhibitor Dickkopf-1 (DKK1) to increase melanogenesis in normal human melanocyte ex vivo [91, 92]. Interestingly, the results from animal models present a paradoxical outcome. These contradictory results may be due to heterogeneity between cells and different concentrations of SP.
It is well-established that VIP, an immunoinhibitory neuropeptide secreted from perifollicular sensory nerve endings, prevents the HFs from IP collapse [93] and promotes melanogenesis in the B16F10 cell line and normal human melanocytes basically by activating the PKA/CREB/MITF signaling pathway [94].
Adipose tissue
It has been shown that dermal white adipose tissue (dWAT) is located underneath and partially integrated into the reticular dermis, surrounding HFs [95]. Interestingly, there is a strong interaction between HFs and dWAT [96]. HFs drive a cycle of dWAT remodeling, where HFs secrete adipogenic activators at the beginning of new hair growth, and at the end of hair growth, activator secretion decreases or adipogenesis inhibitor secretion increases, leading to lipolysis [95, 97]. dWAT is rich in growth factors that signal reciprocally to HF and regulates the activation state of their stem cells and the rate of hair regeneration [95].
Importantly, mature dWAT can inhibit hair growth. During early telogen, HFs enter a refractory phase to growth signaling, partly mediated by bone morphogenetic protein 2 (BMP2) expressed by adjacent dermal adipocytes [97]. A large area of dWAT expresses BMP2, which highly maintains the quiescence of HFSC to prevent excessive hair production [95].
In contrast, dWAT in anagen positively affects hair growth. Adipose progenitor cell is essential for HF to enter a new anagen and is widely thought to stimulate telogen HFs through high levels of platelet‐derived growth factor alpha (PDGFA) [95]. Hepatocyte growth factor (HGF), secreted by anagen perifollicular dWAT, stimulates Wnt/β-catenin signaling in the hair matrix by inhibiting Wnt antagonist frizzled-related protein 1 (SFRP1) as well as upregulating WNT10B, thereby promoting melanocyte maturation and pigmentation [98].
Current evidence suggests that dWAT plays significant roles in the aging process of HF [99]. Aging dWAT represses Wnt signaling by upregulating Wnt inhibitors dickkopf-related protein (DDK) and SFRP4. Additionally, there is an increase in the expression of fibroblast growth factor (FGF)5 and BMP2, which inhibit hair growth, while the secretion of hair growth-promoting factors such as FGF10 and FGF7 is reduced. Compared with young dWAT, senescent dWAT in telogen expresses abundant abnormal inflammatory factors. In aging dWAT during anagen, the inflammatory process is largely suppressed, but collagen production, angiogenesis, and melanogenesis are impaired.
Given that melanogenesis and hair growth share common signaling pathways, it is highly conceivable that dWAT regulates hair graying, although further research is warranted to validate this hypothesis and explore the underlying mechanisms.
Adiponectin, an adipocyte hormone, is specifically and abundantly expressed in WAT. Adiponectin or activation of adiponectin receptor 1 (AdipoR1) can upregulate multiple hair growth factors through AMP-activated protein kinase (AMPK) in human follicular DPC, including IGF-1, vascular endothelial growth factor (VEGF), IGF, HGF, PDGFA, and FGF 7, and downregulate TGFβ1, thereby inducing the anagen and promoting hair growth ex vivo and in vivo [100-103]. In contrast, ex vivo studies revealed that adiponectin oligomer downregulates pigmentation genes in HF and important factors such as Wnt10B and the HGF receptor c-Met within the hair matrix and DP [103]. Meanwhile, neutralizing adiponectin isoforms within HF and dWAT promotes melanocyte proliferation, melanogenesis, and tyrosinase activity but produces fewer melanocytes and dendrites. Nevertheless, hair matrix keratinocyte proliferation and hair pigmentation were not altered by adiponectin oligomer within 48h ex vivo.
Adiponectin exists in two forms in circulation, a full-length protein and a fragment containing the globular domain of adiponectin (gAd). The full-length adiponectin induces depigmentation via AMPK/ CREB-regulated transcription coactivators (CRTCs)/CREB signaling pathway [104], but the gAd promotes melanogenesis by activation of the AMPK-p38 MAPK-CREB pathway [105]. Hence, the proportion of adiponectin oligomer to globular adiponectin or HGF to adiponectin could determine the quantity of synthesized melanin.
Adipose-derived stem cells (ADSCs) are an important type of stem cell that can be isolated from adipose tissue. They are characterized by their ability to differentiate into multiple cell types, ease of availability, high proliferative capacity, and self-renewal potential [106]. ADSCs exert their multiple regulatory roles mainly through autocrine and paracrine pathways [107].
Besides producing various cytokines that promote hair growth, such as VEGF, PDGF, and HGF [108], ADSCs also secrete exosomes to regulate HF. An increasing body of evidence suggests that adipose-derived stem cell exosome (ADSC-Exo) enhance DPC proliferation and survival and promote hair regeneration, mediated in part by inhibiting TGF-β/SMAD3 signaling by miR-122-5p carried in ADSC-Exo [109-111]. Moreover, ADSC-induced amphiregulin promotes hair regeneration of skin-derived precursors (SKPs), a multipotent precursor cell population from the dermis capable of differentiating into several lineages through activation of PI3K and MAPK pathways [112]. Stromal vascular fraction (SVF), the regenerative cell cocktail obtained mainly from ADSCs, has also been shown to treat alopecia areata effectively and safely [113].
In addition to promoting hair growth, ADSCs are also a regulator of melanogenesis. Interestingly, ADSCs can inhibit the proliferation and melanogenesis of epidermal melanocytes through an interleukin-6 (IL-6)-mediated mechanism [114] and through upregulation of TGF-β1 [43]. UVB-induced skin pigmentation is reduced in the area of the skin where ADSCs have been injected, possibly related to the fact that α-MSH/MCIR/cAMP signaling is suppressed by basic fibroblast growth factor (bFGF) secreted from ADSCs [115-117]. It also has been reported that SVF can inhibit UVB-induced pigmentation in guinea pig skin [118].
Intriguingly, ADSCs have shown the potential to promote pigmentation in the context of vitiligo treatment. ADSCs not only promote the proliferation and migration of co-cultured melanocytes and reduce their differentiation [119] but also improve the effectiveness of melanocyte transplantation for vitiligo, likely because ADSCs upregulate bFGF and SCF and then increases the expression of integrins in melanocyte [120, 121]. Ex vivo, adipose tissue extracellular fraction (AT-Ex) induces melanocyte intracellular antioxidant enzymes via acting on nuclear factor (erythroid-derived 2) -like (Nrf-2) to counteract oxidative stress, promotes cell proliferation, and inhibits GSK3β to activate Wnt/β-catenin signaling [122]. Similarly, mice models revealed that NB UVB/ADSCs transplantation combination therapy could improve oxidative stress and calcium homeostasis by stimulating Nfr2/ heme oxygenase (HO -1) signaling, causing vitiligo repigmentation [123].
While there are some inconsistencies in the studies mentioned above, it is plausible to speculate that ADSCs may promote the proliferation and survival of melanocytes by reducing inflammation and maintaining melanocyte quiescence.
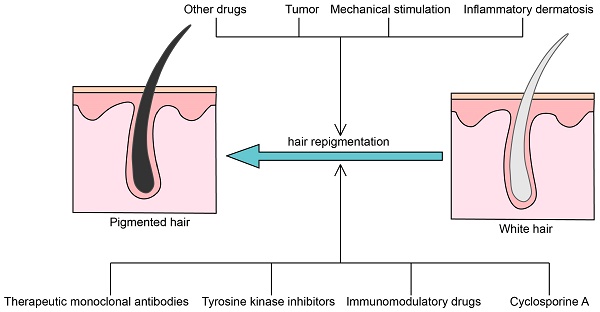
Cases of gray hair repigmentation and possible mechanisms
Therapeutic monoclonal antibodies
Monoclonal antibodies (mAbs), which are immunoglobulins, can target a specific epitope on an antigen and have emerged as an important class of therapeutic drugs. To date, numerous mAbs have received marketing approval [124].
A cell surface receptor called programmed death-1 (PD-1) acts as a T cell checkpoint and is critical in controlling T cell exhaustion. When PD-1 binds to its ligand programmed death ligand 1 (PD-L1), this triggers a downstream signaling pathway that suppresses T cell activation.
Tumor immune evasion is mediated by abnormally elevated PD-L1 expression on tumor cells and antigen-presenting cells in the tumor microenvironment [125]. Anti-PD-1/PD-L1 antibodies, one of the immune checkpoint inhibitors (ICIs), restore the immune response to cancer cells by rescuing T cells from an exhausted state, which have been approved for treating multiple malignancies [126].
A series of 14 patients undergoing anti-PD1/anti-PD-L1 therapy for lung cancer demonstrated hair repigmentation, suggesting it is a promising indicator of positive treatment response [127]. Besides, hair repigmentation of the entire body was reported in a male patient with concomitant advanced colorectal cancer and Hodgkin lymphoma who underwent nivolumab treatment, an anti-PD-1 antibody [128]. It is widely thought that PD-1/PD-L1 immunotherapy and cytotoxic tumor destruction cause an inflammatory state, which results in the collapse of the immune privilege of HF and hair repigmentation [129]. It has also been reported that melanogenesis-related genes and melanin production in B16F10 cells was downregulated by PD-L1 from polyinosinic-polycytidylic-treated HaCaT cell [130], which suggests the direct relationship between PD-1/PD-L1 and melanogenesis. However, vitiligo is a common immune-related adverse event in ICIs treatment for melanoma patients [131], possibly due to the induction of an anti-melanocyte response, which has not been observed in lung cancer patients [132]. Moreover, positive staining of anti-PD-L1 antibodies was found in a canities subita patient who experienced extreme trauma [133]. Considering these contradictory evidences, more research must be done to clarify the confusing relationship between melanogenesis and PD-1/PD-L1.
Dupilumab, a monoclonal antibody for interleukin 4 (IL-4) receptor alpha subunit, blocks the IL-4/IL-13/IL-4R axis and reduces T helper 2 (Th2) cell response effectively [134]. Hair repigmentation has been reported in an atopic dermatitis patient treated with dupilumab [135]. It has been revealed that IL-4 suppresses the expression of MITF, TYRP-1, and DCT through the Janus Kinase 2 (JAK2)/ Signal Transducer And Activator Of Transcription 6 (STAT6) signaling pathway and then inhibits melanogenesis in human normal melanocytes (HNMs) [136].
As a tumor necrosis factor (TNF) inhibitor, adalimumab is indicated to treat inflammatory disorders, including psoriasis, rheumatoid arthritis, and inflammatory bowel disease. There has been a reported case of hair repigmentation in a rheumatoid arthritis patient treated with adalimumab [137].
TNF inhibits melanogenesis and the viability of melanocytes through multiple pathways and is intricately connected with the pathogenesis of vitiligo [138]. It has been revealed that both TNF and IL-17 treatment of melanocytes downregulated c-KIT, MC1R, MITF, DCT, and other melanogenesis-related genes, and the levels of tyrosinase and melanin significantly decreased [139]. The combined action of TNF and IL-17 has been shown to inhibit melanogenesis through the PKA and MAPK signaling pathways [140]. TNF and IL-17 have been found to induce the expression of β-defensin 3 in cultured keratinocytes. Acting as an antagonist for MC1R, β-defensin 3 can inhibit the activation of adenylate cyclase and tyrosinase induced by α-MSH [139]. Other in vitro studies reveal that TNF-α could reduce melanocyte-stimulating hormones receptor (MSH-R) binding activity, MC1R expression, and the expression of a melanosomal protein gp87, promoters of melanogenesis [141, 142]. IL-6, an inhibitor of melanogenesis, was significantly elevated in human normal human melanocytes treated with TNF-α, IL-17, and interferon-gamma (IFN-γ) [136, 138]. In vitro, IL-6 decreases melanogenesis by reducing the transcription of MITF in melanocytes [143, 144] and blocking the paracrine function of keratinocytes and fibroblasts through the IL-6 / STAT3 / FGF2 pathway [145].
In vitro, the expression of intracellular adhesion molecule-1 (ICAM-1) in melanocytes can be upregulated by TNF-α, which may promote T cell/melanocyte adherence and immunologic cytotoxic damage, resulting in vitiligo [138, 146]. Ample evidence suggests that TNF-α treatment increases ROS in melanocytes and leads to melanocyte toxicity [138, 146].
However, due to the high complexity of the body, TNF may have dual effects on melanogenesis. There is an increasing consensus that TNF-α can stimulate endothelins (EDNs) and SCF secretion from melanocytes and keratinocytes to cause skin hyperpigmentation [147-149]. Additionally, TNF-α-induced production of reactive oxygen species (ROS) contributes to melanogenesis [150]. It is widely thought that Adalimumab may mitigate the high level of TNF-α in rheumatoid arthritis patients, resulting in hair repigmentation, but the underlying mechanism warrants further research (Fig. 2).
The biological roles of inflammatory factors in melanogenesis. Inflammatory factors including PGE2 and PGF2α promote melanogenesis by stimulating cAMP pathway. While IL-4 and IFN-γ inhibit melanogenesis through JAK-STAT pathway. TNF-α inhibits melanogenesis by suppressing the PKA, MAPK and MC1R pathways in combination with IL-17. IL-1β inhibits melanogenesis through NF-κB, JNK and MAPK pathways. IL-6, elevated via TNF-α and IFN-γ, decreases melanogenesis by reducing the transcription of MITF in melanocytes. IL-4: interleukin 4; JAK: Janus Kinase; STAT: Signal Transducer And Activator Of Transcription; IFN-γ: Interferon gamma; IL-6: interleukin 6; PKA: cAMP-dependent protein kinase A; MAPK: mitogen-activated protein kinase; IL-17: interleukin 17; TNF-α: tumor necrosis factor alpha; MC1R: Melanocortin 1 Receptor; PGF2α: Prostaglandin F2α; PGE2: prostaglandin E2; cAMP: cyclic adenosine monophosphate; NF-κB: nuclear factor-κB; JNK: c-Jun N-terminal kinase; IL-1β: interleukin-1β; MITF: Melanocyte Inducing Transcription Factor; TRP-1: Tyrosinase related protein-1; TRP-2: Tyrosinase related protein-2.

Hair pigmentation was observed in a patient with plaque psoriasis undergoing treatment with secukinumab [151]. Secukinumab is a fully human monoclonal antibody that targets interleukin-17A and is utilized in the treatment of various autoimmune diseases.
In addition to its synergistic effects with TNF, IL-17 suppresses melanogenesis through ROS-dependent autophagic melanocyte apoptosis and stimulates keratinocytes to secrete IL-1β through the nuclear factor-κB (NF-κB)/ROS/ NLR Family Pyrin Domain Containing 3 (NLRP3)/caspase 1 pathway [152]. Similarly, the production of TNF-α and IL-6 in keratinocytes and fibroblasts is increased dramatically by IL-17A ex vivo [153]. Further investigation is needed to determine the actual impact of IL-1β on melanogenesis when it directly interacts with melanocytes. In melanoma cell lines, IL-1β has been shown to downregulate MITF expression through the activation of NF-κB, c-Jun N-terminal kinases (JNKs), and microRNA-155 [154, 155]. Additionally, IL-1β has the ability to induce apoptosis in melanocytes [156]. However, contrasting findings have also been observed. It has been discovered that IL-1β can upregulate MC1R expression in normal human melanocytes [142]. Furthermore, recent research has indicated that IL-1β partially mediates UVB-induced melanogenesis by upregulating the expression of TYR and TRP1 in melanoma B16 cells [157]. Nevertheless, it is unquestionable that both IL-17 and IL-1β are positively associated with the progression and extent of vitiligo, likely due to autoimmune melanocyte death [158-161].
Interestingly, an increase in melanocyte abundance and a concomitant decline in pigmentation signaling was observed in psoriasis lesions known to overexpress IL-17 and TNF [139]. It has been reported that speckled lentigines appeared in resolved psoriatic plaques after treatment with biological agents, such as scukinumab[162, 163]. The occurrence of lentigines may be attributed to the removal of inhibition on melanogenesis when TNF-α and IL-17 are blocked. When these inhibitory factors are suppressed, melanocytes in resolved lesions may produce excessive melanin, leading to the formation of lentigines. The mechanism of hair repigmentation following treatment may share similarities with the development of skin lentigines.
Ustekinumab, an anti-interleukin IL-12/23 p40 monoclonal antibody, induced hair repigmentation in a psoriasis vulgaris patient [164]. It is widely believed that activation of the TH17 pathway by IL-23 is the predominant mechanism involved in the pathogenesis of psoriasis. The survival and proliferation of TH17 and TH22 cells depend on IL-23. IL-17, IL-22, and TNF-α are produced by TH17 cells, and IL-22 is produced by TH22 cells. Naive T cells are converted into TH1 cells by IL-12, which secretes IFN-γ and TNF-α [165]. Ustekinumab may lead to hair pigmentation by decreasing these inhibitors of melanogenesis (IL-17 and TNF-α).
Brentuximab vedotin is an antibody-drug combination commonly used to treat CD30+ lymphomas. This conjugation binds to the CD30 antigen on the surface of cells expressing CD30. Upon absorption by the cell, the compound undergoes proteolytic cleavage, releasing monomethyl auristatin E. Interestingly, a patient who underwent allogeneic hematopoietic stem cell transplantation and received brentuximab vedotin as a trial for refractory chronic graft-versus-host disease (cGVHD) experienced hair repigmentation [166]. CD30 signaling enhances the activation of TH1 and TH17 cells, leading to increased production of INF-γ and IL-17A. Additionally, CD30 signaling promotes the secretion of TNF-α and IL-6 through the activation of NF-κB [167, 168]. Importantly, these cytokines (INF-γ, IL-17A, TNF-α, and IL-6) are known to negatively regulate melanogenesis. The observed hair repigmentation induced by brentuximab may be attributed to the elimination of these proinflammatory cytokines, which allows for the restoration of normal melanogenesis processes.
Tyrosine kinase inhibitors
In a retrospective cohort study involving 133 chronic myeloid leukemia (CML) patients treated with imatinib, 9 cases of hair repigmentation were reported [169]. Hair repigmentation has also been observed in a nilotinib-treated CML patient [170]. Several cases of hair pigmentation following sorafenib and erlotinib treatment for lung adenocarcinoma have been documented [171, 172]. Indeed, all these drugs belong to the class of tyrosine kinase inhibitors.
As the first protein tyrosine kinase inhibitor, imatinib inhibits Bcr-Abl, platelet-derived growth factor receptor (PDGFR), and c-Kit. It has been approved for treating Philadelphia-chromosome-positive CML and gastrointestinal stromal tumors (GIST) [173]. Nilotinib and dasatinib are second-generation inhibitors developed to address imatinib resistance in CML [174]. In recent years, alternative mechanisms of nilotinib have been discovered, such inactivation of p38 MAPK in microglial/astroglial cells and a myoblast cell line [175, 176], inhibition of the discoidin domain receptor (DDR) in metastatic colorectal cancer cells [177], and prevention of NF-κB activation in microglial cells [178] indicating there are more target points of nilotinib. It has been reported that nilotinib and dasatinib promote melanogenesis in vitro. In HM3KO melanoma cells, nilotinib was found to upregulate MITF and its downstream genes by activation of the cAMP/PKA/CREB signaling pathway and decreasing the phosphorylation of AKT, which repressed the pigmentation process by inhibition of GSK3β [179]. In B16F0 mouse melanoma cells, nilotinib was found to increase ROS levels and ROS-induced JNK activation, thereby inducing TYR, TRP1, and TRP2 [180]. Dasatinib has also been discovered to promote melanogenesis in human normal melanocytes through ERK/CREB/MITF signaling and possibly through phosphorylation of p38 MAPK and JNK [181].
Sorafenib is a multiple-target tyrosine kinase inhibitor, inhibiting Raf1, VEGF receptors, platelet-derived growth factor (PDGF) receptors, and several other targets [182]. It has been shown that Sorafenib also upregulates MITF and melanogenesis in the HM3KO melanoma cell line by repression of AKT and ERK pathway and increase of β-catenin via reduction enzyme activity of GSK3β [183].
It has been established that erlotinib selectively inhibits epidermal growth factor receptor (EGFR) and can be used to treat several solid tumors [184]. Although EGFR signaling negatively affects UVR-induced melanogenesis [185, 186], it is highly conceivable that post-inflammatory hyperpigmentation is caused by erlotinib-induced follicle inflammation [171].
It has been established that tyrosine kinase inhibitors (TKIs) have various cutaneous adverse effects, with skin and hair depigmentation being relatively common but hair repigmentation occurring less frequently [187-189]. The exact mechanism by which TKIs promote hair repigmentation is not yet fully understood, although it is believed to be due in part to their ability to enhance melanogenesis.
Immunomodulatory drugs
Two cases have been reported in which multiple myeloma (MM) patients experienced hair repigmentation after receiving lenalidomide or thalidomide treatment [190, 191]. These drugs, which are immunomodulatory drugs (IMiDs), are approved for specific types of hematological cancers and autoimmune diseases. Protein cereblon (CBRN) is a member of the Lon protease family that plays a crucial role in mediating the anti-myeloma effects and teratogenicity of this class of IMiDs [192]. IMiDs have been shown to decrease the production of TNF-α in human peripheral blood mononuclear cells (PBMCs) by promoting the degradation of TNF-α mRNA, potentially through CBRN [192]. Additionally, IMiDs have been demonstrated to reduce the expression and secretion of IL-6, which is known to be upregulated by TNF-α [193]. IMiDs also produce therapeutic effects on several diseases by inhibiting TGF-β [194-198]. It has also been reported that IMiDs reduce IL-1 and IL-1β in plasma [199]. What's more, IMiDs inhibit the activation of NF-κB by blocking the degradation of inhibitor of NF-κB (IκB) proteins [200].
TNF-α, IL-6, TGF-β, and IL-1β are all recognized as inhibitors of melanogenesis [201]. The impact of TNF-α and IL-6 on melanogenesis was previously examined in the context of "Adalimumab," while the influence of IL-1β was discussed in relation to "Secukinumab."
Moreover, it has been established that IL-10, which activates the STAT-3 and PI3K/AKT/NF-κB signaling pathways to protect primary melanocytes [202], is induced by IMiDs [192, 203].
NF-κB signaling participates in both the stimulation and the suppression of melanogenesis. It was reported that TNF-α, tumor necrosis factor superfamily member 14 (TNFSF14), and IL-1β induce melanogenesis by activating NF-κB [204, 205]. However, other studies suggest that activation of NF-κB mediates the facilitation of melanogenic activity from multiple sources, such as IL-18, Toll-like receptor 9 agonists, and UVR-induced-oxidative stress, indicating that the target genes involved in regulating melanogenesis may not be identical when NF-κB signaling is activated [206-208].
Interestingly, in multiple myeloma patients, IMiDs increase the production of IFN-γ (an inhibitor of melanogenesis) from T cells, and IMiDs downregulate VEGF, bFGF, and granulocyte macrophage-colony stimulating factor (GM-CSF), all of which have promotive effects on melanogenesis [193, 201, 209-213]. The hair repigmentation effects of IMiDs are primarily attributed to their ability to inhibit the elevated levels of melanogenic inhibitors observed in multiple myeloma patients.
Cyclosporine A (CsA)
Cyclosporine A, the first reported immunosuppressive drug to selectively inhibit T cells, mainly targets Th cells to achieve its therapeutic effect [214]. CsA has been shown to induce hair repigmentation in psoriasis patients [215-217]. HF is an immediate target of CsA, and hypertrichosis may be the most intriguing and most common adverse effect of CsA [218]. In vitro, CsA downregulates SFRP1 in DP, an inhibitor of the Wnt ligand, which activates the Wnt/β-catenin pathway in HF and then induces hair growth [219]. Therefore, cyclosporine may promote melanogenesis by activating the WNT pathway in melanocytes. However, it is possible that CsA's immunosuppressive properties and its ability to reduce cytokine levels could contribute to hair repigmentation by inhibiting melanogenesis.
Other drugs
A study revealed that hair repigmentation occurred in two patients (one case is myxedema coma, and the other is iatrogenic hyperthyroidism) after receiving high-dose thyroxine treatment [220]. In organ-cultured normal human scalp HFs, TH enhances the proliferation of hair matrix keratinocytes, inhibits their apoptosis, and induces and prolongs the anagen phase through the downregulation of TGF-β2, a key catagen-promoting growth factor [220, 221].
It has been reported that T4 downregulates the intrafollicular expression of clock genes (BMAL1 and PER1) after 24h, both of which inhibit anagen/prolong catagen and inhibit HF pigmentation [222]. Both T3 and T4 significantly promote melanogenesis of organ-cultured HF, the mechanism of which possibly is independent of the hair cycle [221]. Thyroid hormone signaling is related to many pigmented dermatoses and hair disorders, such as vitiligo, melanocytic nevi, and alopecia areata [223, 224], indicating that thyroxine plays an important role in hair pigmentation and maintaining the homeostasis of melanocytes.
Hair repigmentation was induced in a glaucoma patient treated with latanoprost, a prostaglandin F2α (PGF2α) analog [225]. It is now understood that iris pigmentation, eyelashes hypertrichosis, and hyperpigmentation are common adverse events [226]. It has been revealed that PGF2α and prostaglandin E2 (PGE2) promote melanocyte dendrites formation and the activation of tyrosinase through cAMP/PLC signaling [227-230]. Moreover, ex vivo, PGE2 promotes the delivery of filopodia and quantities of shedding spheroid granules in melanocytes (MCs) but does not influence the morphology of keratinocytes [231]. Current evidence suggests that local application of latanoprost activates HFs and encourages hair growth, and bimatoprost, another PGF2α analog, has been approved for treating eyelash hypotrichosis [232, 233]. A murine model validated the stimulatory effect of PGF2α and latanoprost on follicular melanogenesis and hair regrowth [234].
A retrospective study reported that hair repigmentation occurred in 24 of the 62 Alzheimer's patients receiving prolonged cholinesterase inhibitor therapy [235]. Solar light not only induces skin keratinocytes to secrete acetylcholine (ACh), which represses light-induced melanogenesis probably by inhibiting cAMP/CREB/MITF signaling in melanocytes [236, 237] but also promotes expression of AChE in keratinocytes through transcription factor activator protein 1 (AP1) ex vivo [238]. What's more, during melanin production in melanocytes and B16F10 melanoma cells, AChE is downregulated by increased cAMP/CREB signaling [237]. The above findings suggest that ACh and AChE inhibitors are local negative regulators of melanogenesis, and there is a negative feedback loop of ACh-melanogenesis-AChE among melanocytes and keratinocytes to maintain melanin homeostasis in the skin.
Nevertheless, it was recently reported that phagocytosis mediated by the α7 nicotinic acetylcholine receptor causes the skin keratinocyte to take up melanosomes in response to UV exposure in vitro [239]. Moreover, M4 receptor-KO mice exhibit poor hair growth with no HF melanogenesis [240], indicating that cholinergic signaling is indispensable for pigmentation.
It has been established that these AChE inhibitors work by increasing ACh in the nervous system to treat patients with Alzheimer's disease. Several reports revealed that ACh enhances the release of α-MSH from pituitary melanotropes and nerve-induced pigmentation in lower vertebrates [241]. In a similar vein, it is also possible that ACh causes hair repigmentation in a neuroendocrine-dependent manner. Meanwhile, considering cholinergic signaling has multiple effects on neural and intestinal stem cells [242], ACh likely plays a role in McSC homeostasis.
A case report has documented that hair repigmentation occurred in a breast cancer patient who received treatment with tamoxifen, the first selective estrogen receptor modulator, for 2.5 years [243]. Although there are conflicting reports, estrogen is considered to have ER-mediated promotive effects on melanogenesis in melanocyte ex vivo, [244] possibly by activation of the cAMP/PKA/MITF pathway [245, 246]. Estrogen also induces melanogenesis indirectly via keratinocytes [244]. In vivo, high estrogen levels enhance melanogenesis and cause skin hyperpigmentation, such as melasma [247]. However, in vitro, tamoxifen was found to stimulate synthesis and extrusion of melanin, with decreased cAMP but upregulated catalase expression in normal human melanocytes, suggesting tamoxifen has a ROS-mediated promelanogenic effect on melanocytes [248]. In practical terms, one of the established anti-cancer mechanisms of tamoxifen involves increasing apoptosis through the generation of reactive oxygen species [249].
L-DOPA has been reported to induce hair pigmentation in 3 patients with Parkinson's disease [250]. In addition to being the intermediate of melanogenesis, L-DOPA is also a bioregulatory molecule that positively regulates melanogenesis by upregulating TYR and MC1R and regulating several cellular processes, such as cellular metabolism [251]. These can be mediated by interacting with certain receptors or by non-receptor mechanisms.
Nevertheless, a study revealed that dopamine directly induced catagen in human scalp HFs ex vivo [252]. Thus, L-DOPA may not be a good choice for beauty lovers who desire pigmented hair.
Cerebrolysin is a low-molecular-weight neuropeptide obtained from the porcine brain and has neuroprotective and neurotrophic effects similar to neurotrophic growth factors. It has been reported that cerebrolysin causes hair repigmentation linked to Melan-A, also known as MART-1 reactivation, in 5 neurological patients [253]. Besides, some neuropeptides, such as CGRP, SP, and VIP, enhance melanocyte proliferation and melanogenesis [80, 91, 94]. In addition, the p75 neurotrophin receptor (p75NTR) regulates apoptosis in the external root sheath of the HF [254]. Therefore, cerebrolysin may cause hair repigmentation as a neurotrophin factor. Meanwhile, given that melanocytes are derived from the neural crest, the neuroprotective effects of cerebrolysin, which include preventing nerve cell apoptosis and promoting differentiation and migration, may also be beneficial for melanocytes [253].
As second-generation retinoids, etretinate and acitretin exert their effects by binding to retinoic acid receptors and retinoid-X receptors. Interestingly, in a study involving four patients, both etretinate and acitretin were found to induce hair repigmentation and curling [255-258]. It has been demonstrated that an increased level of retinoic acid (RA) upregulates the C-KIT receptor and then sensitizes McSCs to KIT-ligand, eventually leading to ectopic McSCs differentiation in the niche in vivo [19]. Although the mechanism behind hair color change induced by etretinate and acitretin is not completely understood, it is possible that their impact on retinoic acid metabolism could be an alternate mechanism. However, current research only suggests that etretinate and acitretin increase the telogen phase of the hair cycle and inhibit melanocyte proliferation through retinoid-X receptor signaling [259, 260].
According to some reports, some plant extracts have ability to prevent hair graying. Eriodictyon angustifolium (Ea) is a plant that grows on the west coast of North America and has been used for many years as a traditional medicinal herb by the indigenous population. Abundant flavonoids contained in Ea extract, such as sterubin and hydroxygenkwanin, seem to be active ingredients to prevent and reduce hair graying [261, 262]. Although the target is not molecularly clear, sterubin appears to function by activating Wnt signal and reducing ROS [263], while hydroxygenkwanin relies on KIT signaling [264]. Polygonum multiflorum(PM) is a tranditonal Chinese medicine that has been experiencely used to treat early graying hair for a long time. PM extract has been proven to potentiate melanin synthesis by targeting on α-MSH and MC1R and reducing ROS production [265-267]. Besides Ea and PM, Pueraria lobata extract and its active compound, puerarin, also have been reported to prevent hair graying via the cAMP/MITF signaling pathway [268, 269].
Micro-injury
Hair repigmentation was observed in an 84-year-old woman after Mohs micrographic surgery and secondary intention wound healing [270]. Physical therapy, such as phototherapy and microneedle, has been used to treat pigmentation disorders and hair loss for years [271-273]. In response to injury or UVB, HF-McSCs migrate to the epidermis, depending on MC1R signaling. Then they differentiate to produce protective pigmentation against UV [30]. Except for the absence of pigmentation, de novo regenerated hair follicles can't be distinguished from regularly growing hair follicles. However, when mice are injured during the anagen, de novo regeneration of pigmented hair is seen. This phenomenon may be caused by the fact that, stimulated by increased Wnt7a of the keratinocytes, McSCs that have been induced to migrate to the interfollicular epidermis by injury are integrated into de novo regenerated hair follicles [274]. Moreover, activation of the β-Catenin signaling pathway, at least in part, in melanocyte stem cells located in the hair follicle bulge area is responsible for the narrowband UVB (NBUVB)-induced repigmentation of vitiligo [275]. Recent studies have also reported that the Wnt/β-Catenin pathway is involved in hair regeneration and vitiligo repigmentation following micro-injury [276].
As a form of injury, epilation activates McSCs to regenerate follicular and epidermal melanocytes through induced endothelin 3/endothelin type-B receptor (EDN3/EDNRB) signaling, leading to skin and hair hyperpigmentation [277].
Overall, the mechanism of micro-injury-induced hair repigmentation is closely related to the Wnt/β-Catenin and EDN3/EDNRB pathways.
Tumor
Roughly ten cases have been reported documenting focal hair repigmentation occurring on and around the area of scalp melanoma [278-287]. A hypothesized model for this phenomenon involves two key cascades. The first cascade involves infiltrating melanoma cells, directly delivering melanin to follicular keratinocytes [283, 287]. However, histopathological examination revealed that many hair follicles remain uninvaded by melanoma cells but still exhibit repigmented hair. This leads to the second cascade, where benign bulbar melanocytes are activated by paracrine factors (such as SCF) released by neighboring melanoma cells [283, 287]. Immunostaining studies have confirmed the presence of TGF-β1-expressing follicular epithelium adjacent to highly TGF-β1-positive melanoma cells [287], supporting this second cascade.
It has been reported that paraneoplastic syndrome caused by lung cancer could induce the darkening of hair and skin in several patients. The potential mechanism may be that a high level of ACTH in the body promotes melanogenesis through the MC1R signaling pathway [1, 288] (Table 1).
Conclusion
While widespread repigmentation of gray hair is uncommon, the underlying mechanism remains an important area of study. Understanding the regulatory mechanisms involved in hair follicle melanogenesis and melanocyte stem cells is essential for developing potential clinical therapies to reverse gray hair. However, achieving this goal necessitates extensive research efforts. Given that the skin is an accessible part of the body and the position of the HFs is relatively superficial, topical treatment will have great advantages in safety and effectiveness.
Cases of gray hair repigmentation and possible mechanisms
| Cases | possible mechanisms | |
|---|---|---|
| Monoclonal antibody drug | Anti-PD-1/PD-L1 therapy | An inflammatory state caused by PD-1/PD-L1 immunotherapy and cytotoxic tumor destruction results in the collapse of the immune privilege of the hair follicle and promotes hair repigmentation. |
| Dupilumab | It removes IL-4/JAK2/STAT6 signaling pathway's inhibition of melanogenesis. | |
| Adalimumab | It blocks TNF-α and IL-17 signaling and then gets rid of inhibition on melanogenesis. | |
| Scukinumab | It blocks TNF-α and IL-17 signaling and gets rid of inhibition on melanogenesis. | |
| Ustekinumab | It decreases TNF-α and IL-17 produced by TH17 and TH22 cells. | |
| Brentuximab | It blocks CD30 signaling in TH1 and TH17 and then decreased INF-γ, IL-17A, TNF-α and IL-6. | |
| tyrosine kinase inhibitors | Nilotinib | It increases melanogenesis through activation of cAMP/PKA/CREB/MITF signaling pathway and decreases AKT signaling. It also elevates ROS and induces JNK activation to upregulate TYR, TRP-1, TRP-2. |
| Dasatinib | It promotes melanogenesis through MAPK or JNK/ERK/CREB/MITF signaling. | |
| Imatinib | The reason may be similar to Nilotinib and Dasatinib's. | |
| Sorafenib | It represses AKT and ERK pathway and increases β-catenin. | |
| Erlotinib | Post-inflammatory hyperpigmentation after erlotinib-induced follicle inflammation. | |
| Immunomodulatory drugs | lenalidomide or thalidomide | They decrease inhibitors of melanogenesis and increase promotors. |
| Immunosuppressant | Cyclosporine A | CsA downregulates SFRP1, an inhibitor of Wnt ligand, which in turn activates Wnt/β-catenin pathway and then induces hair repigmentation. |
| Other drugs | L-thyroxine | TH inhibits TGF-β2 and promotes melanogenesis. BMAL1 and PER1 are downregulated to prolong anagen. |
| Latanoprost | PGE2 and PGF2α stimulate melanogenesis by cAMP/PLC signaling and promoting melanosome delivery in melanocyte. Promotes hair follicle growth. | |
| Acetylcholinesterase inhibitor | Not clear but cholinergic signaling is necessary for melanogenesis. Ach also seems to increase α-MSH secretion and regulate stem cells. | |
| Tamoxifen | It induces ROS in melanocyte to stimulate melanogenesis. | |
| L-DOPA | It upregulates TYP and MC1R to enhance melanogenesis. | |
| Cerebrolysin | As a kind of neuropeptide, it causes melanogenesis linked to Melan-A as a kind of neuropeptide | |
| Retinoids | The impact of etretinate and acitretin on RA metabolism, which upregulates C-KIT receptor and leads to ectopic McSCs differentiation, may be an alternate mechanism for hair color change | |
| Mechanical stimulation | Micro-injury | It activates MC1R signaling, Wnt signaling and EDNRB signaling and then promotes melanogenesis. |
| Tumor | Scalp melanoma | Melanoma cells directly deliver melanin to keratinocytes or secrete some factors to activate surrounding normal melanocyte in a paracrine manner. |
| Lung cancer | Paraneoplastic syndrome in lung cancer leads to high level of ACTH, which can stimulate MC1R signaling. |
Just as topical minoxidil is commonly used to treat hair loss, topical agents that stimulate melanin synthesis to reverse gray hair will have promising prospects if they can be developed in the future. Physical therapies such as photodynamic therapy and microneedling are also viable options. However, all these treatments rely on the presence of a sufficient population of active McSCs. Therefore, maintaining a healthy population of McSCs is also an urgent problem that needs to be addressed.
Abbreviations
TKIs: tyrosine kinase inhibitors; HF: hair follicle; DOPA: dihydroxyphenylalanine; HFPU: hair follicle pigment unit; McSC: Melanocyte stem cell; ORS: outer root sheath; GSK3β: Glycogen synthase kinase 3β; MITF: Melanocyte Inducing Transcription Factor; EDNRB: Endothelin Receptor Type B; EpSCs: epithelial stem cells; TACs: transit-amplifying cells; MC1R: Melanocortin 1 Receptor; UVB: Ultraviolet B; POMC: pro-opiomelanocortin; α-MSH: α-melanocyte-stimulating hormone; ACTH: adrenocorticotropic hormone; cAMP: cyclic adenosine monophosphate; PKA: protein kinase A; CREB: cAMP responsive element-binding protein; SOCE: Store‐operated Ca2+ entry; ER: endoplasmic reticulum; STIM1: stromal interaction molecule 1; SOC: store-operated Ca2+; PLC: phospholipase C; IP3: inositol triphosphate 3; ADCY6: adenylyl cyclase 6; UVR: Ultraviolet radiation; SCF: stem cell factor; MAPK: mitogen-activated protein kinase; ERK: Extracellular signal-regulated kinase; RSK: ribosomal S6 kinase; MSK1: Mitogen- and stress-activated kinase 1; EDN1: Endothelin 1; PI3K: phosphoinositide 3-kinase; AKT: serine/threonine-specific protein kinase; TGF-β: Transforming growth factor-β; PAX3: paired-box homeotic gene 3; SOX10: SRY-Box Transcription Factor 10; CRE: cAMP-response element; TRP2: tyrosinase-related protein 2; TYR: tyrosinase; TYRP-1: tyrosine-related protein-1; DCT: dopachrome tautomerase, also known as tyrosine-related protein-2, TYRP-2; GPR143: Protein-Coupled Receptor 143; MART-1: Melanoma-associated antigen recognized by T cells; MYO5a: Myosin5a; CDK2: cyclin dependent kinase 2; TBX2: T-box transcription factor 2; CCNB1: cyclin B1; CCND1: cyclin D1; PLK1: polo-like kinase 1; BCL2: B-cell-lymphoma 2; BIRC7: baculoviral IAP repeat containing 7; LIG1: DNA ligase I; TERT: telomerase reverse transcriptase; EME1: essential meiotic endonuclease 1 homolog 1; BRCA1: Breast Cancer 1 protein; FANCA: Fanconi anemia protein A; GTF2H1: General transcription factor IIH subunit 1; TFIIH: transcription factor IIH; ROS: reactive oxygen species; APE1: apurinic-apyrimidinic endonuclease1; HIF1α: hypoxia-inducible factor 1; PGC1α: Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; SHH: Sonic hedgehog; CGRP: calcitonin gene-related peptide; SP: substance P; VIP: vasoactive intestinal peptide; IP: immune privilege; IGF-I: insulin-like growth factor-I; NK1R: neurokinin-1 receptors; NGF: nerve growth factor; p75NTR: apoptosis- and catagen-inducing receptor; TrkA: tyrosine kinase receptor type 1; 5-HT: 5-hydroxytryptamine; S6K1: S6 kinase 1; HPA: hypothalamic pituitary adrenocortical; DKK1: Dickkopf-1; NAS: N-Acetylserotonin; CRS: chronic restraint stress; CUMS: chronic unpredicted mild stress; GABA: Gamma-aminobutyric acid; DPC: dermal papilla cell; dWAT: Dermal white adipose tissue; BMP2: bone morphogenetic protein 2; PDGFA: platelet‐derived growth factor alpha; HGF: Hepatocyte growth factor; SFRP1: frizzled related protein 1; DDK: dickkopf related protein; FGF: fibroblast growth factor; AdipoR1: adiponectin receptor 1; AMPK: AMP-activated protein kinase; VEGF: vascular endothelial growth factor; gAd: globular domain of adiponectin; CRTCs: CREB-regulated transcription co-activators; ADSC-Exo: Adipose-derived stem cell exosome; SKPs: skin-derived precursors; SVF: Stromal vascular fraction; IL-6: interleukin-6; AT-Ex: adipose tissue extracellular fraction; mAbs: monoclonal antibodies; PD-1: programmed death-1; PD-L1: ligand programmed death ligand 1; ICIs: immune checkpoint inhibitors; IL-4: interleukin 4; Th2: T helper 2; EoE: eosinophilic esophagitis; JAK2: Janus Kinase 2; STAT6: Signal Transducer And Activator Of Transcription 6; HNMs: human normal melanocytes; TNF: tumor necrosis factor; MSH-R: melanocyte-stimulating hormones receptor; FGF2: Fibroblast growth factor 2; ICAM-1: intracellular adhesion molecule-1; EDNs: endothelins; NF-κB: nuclear factor-κB; NLRP3: NLR Family Pyrin Domain Containing 3; JNKs: c-Jun N-terminal kinases; cGVHD: chronic graft-versus-host disease; CML: chronic myeloid leukemia; PDGFR: platelet-derived growth factor receptor; GIST: gastrointestinal stromal tumor; DDR: discoidin domain receptor; VEGF: vascular endothelial growth factor; PDGF: platelet-derived growth factor; EGFR: epidermal growth factor receptor; IMiDs: immunomodulatory drugs; CBRN: lon protease Cereblon; PBMSs: peripheral blood mononuclear cells; CRBN: Cereblon; IκB: inhibitor of NF-κB; TNFSF14: tumor necrosis factor superfamily member 14; MM: multiple myeloma; bFGF: basic fibroblast growth factor; GM-CSF: granulocyte macrophage-colony stimulating factor; CsA: Cyclosporine A; INF-α: interferon-α; HFs: hair follicles; TH: thyroid hormone; PGF2α: Prostaglandin F2α; PGE2: prostaglandin E2; MCs: melanocytes; AChE: Acetylcholinesterase; Ach: Acetylcholine; AP1: activator protein 1; L-DOPA: Levodopa; p75NTR: p75 neurotrophin receptor; RA: retinoic acid; HF-McSCs: hair follicle melanocyte stem cells; EDN3/EDNRB: endothelin 3/endothelin type-B receptor; sHG: secondary hair germ; NBUVB: Narrowband UVB; c-KIT: tyrosine kinase receptor.
Acknowledgements
This work was supported by Affiliated Hospital of Xuzhou Medical University.
Author Contributions
ZRF prepared the related literature, and was a major contributor in writing the manuscript. YQ drew the pictures and wrote part of the manuscript. GJ provided direction throughout the preparation of this manuscript, and made significant revisions to the manuscript. All authors have read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. O'Sullivan JDB, Nicu C, Picard M, Cheret J, Bedogni B, Tobin DJ. et al. The biology of human hair greying. Biol Rev Camb Philos Soc. 2021;96:107-28
2. Ji J, Ho BS, Qian G, Xie XM, Bigliardi PL, Bigliardi-Qi M. Aging in hair follicle stem cells and niche microenvironment. J Dermatol. 2017;44:1097-104
3. Park AM, Khan S, Rawnsley J. Hair Biology: Growth and Pigmentation. Facial Plast Surg Clin North Am. 2018;26:415-24
4. He L, Michailidou F, Gahlon HL, Zeng W. Hair Dye Ingredients and Potential Health Risks from Exposure to Hair Dyeing. Chem Res Toxicol. 2022;35:901-15
5. Rosenberg A, Rausser S, Ren J, Mosharov E, Sturm G, Ogden R. et al. Quantitative mapping of human hair greying and reversal in relation to life stress. eLife. 2021 10
6. Fernandez-Flores A, Saeb-Lima M, Cassarino DS. Histopathology of aging of the hair follicle. J Cutan Pathol. 2019;46:508-19
7. Wu X, Hammer JA. Melanosome transfer: it is best to give and receive. Curr Opin Cell Biol. 2014;29:1-7
8. Sun Q, Lee W, Hu H, Ogawa T, De Leon S, Katehis I. et al. Dedifferentiation maintains melanocyte stem cells in a dynamic niche. Nature. 2023
9. Tobin D, Hagen E, Botchkarev V, Paus R. Do hair bulb melanocytes undergo apoptosis during hair follicle regression (catagen)?. The Journal of investigative dermatology. 1998;111:941-7
10. Slominski A, Wortsman J, Plonka P, Schallreuter K, Paus R, Tobin D. Hair follicle pigmentation. The Journal of investigative dermatology. 2005;124:13-21
11. Oh JW, Kloepper J, Langan EA, Kim Y, Yeo J, Kim MJ. et al. A Guide to Studying Human Hair Follicle Cycling In vivo. J Invest Dermatol. 2016;136:34-44
12. Tobin D. A possible role for Langerhans cells in the removal of melanin from early catagen hair follicles. The British journal of dermatology. 1998;138:795-8
13. Tobin D, Slominski A, Botchkarev V, Paus R. The fate of hair follicle melanocytes during the hair growth cycle. The journal of investigative dermatology Symposium proceedings. 1999;4:323-32
14. Commo S, Bernard B. Melanocyte subpopulation turnover during the human hair cycle: an immunohistochemical study. Pigment cell research. 2000;13:253-9
15. Nishimura E, Jordan S, Oshima H, Yoshida H, Osawa M, Moriyama M. et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854-60
16. Commo S, Gaillard O, Bernard B. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. The British journal of dermatology. 2004;150:435-43
17. Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720-4
18. Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S. et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177-87
19. Lu Z, Xie Y, Huang H, Jiang K, Zhou B, Wang F. et al. Hair follicle stem cells regulate retinoid metabolism to maintain the self-renewal niche for melanocyte stem cells. Elife. 2020 9
20. Wu S, Yu Y, Liu C, Zhang X, Zhu P, Peng Y. et al. Single-cell transcriptomics reveals lineage trajectory of human scalp hair follicle and informs mechanisms of hair graying. Cell Discov. 2022;8:49
21. Arck PC, Overall R, Spatz K, Liezman C, Handjiski B, Klapp BF. et al. Towards a "free radical theory of graying": melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006;20:1567-9
22. Takeo M, Lee W, Rabbani P, Sun Q, Hu H, Lim CH. et al. EdnrB Governs Regenerative Response of Melanocyte Stem Cells by Crosstalk with Wnt Signaling. Cell Rep. 2016;15:1291-302
23. Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L. et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941-55
24. Choi BY. Targeting Wnt/beta-Catenin Pathway for Developing Therapies for Hair Loss. Int J Mol Sci. 2020 21
25. Yardman-Frank JM, Fisher DE. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Experimental Dermatology. 2020;30:560-71
26. Li C, Kuai L, Cui R, Miao X. Melanogenesis and the Targeted Therapy of Melanoma. Biomolecules. 2022 12
27. Manning D, Dart C, Evans RL. Store-operated calcium channels in skin. Front Physiol. 2022;13:1033528
28. Motiani RK, Tanwar J, Raja DA, Vashisht A, Khanna S, Sharma S. et al. STIM1 activation of adenylyl cyclase 6 connects Ca(2+) and cAMP signaling during melanogenesis. EMBO J. 2018 37
29. Shah P, He YY. Molecular regulation of UV-induced DNA repair. Photochem Photobiol. 2015;91:254-64
30. Chou WC, Takeo M, Rabbani P, Hu H, Lee W, Chung YR. et al. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med. 2013;19:924-9
31. Ahn JH, Jin SH, Kang HY. LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Arch Dermatol Res. 2008;300:325-9
32. Qian W, Liu W, Zhu D, Cao Y, Tang A, Gong G. et al. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp Ther Med. 2020;20:173-85
33. Hou L, Panthier J, Arnheiter H. Signaling and transcriptional regulation in the neural crest-derived melanocyte lineage: interactions between KIT and MITF. Development (Cambridge, England). 2000;127:5379-89
34. Sato-Jin K, Nishimura EK, Akasaka E, Huber W, Nakano H, Miller A. et al. Epistatic connections between microphthalmia-associated transcription factor and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. FASEB J. 2008;22:1155-68
35. Terazawa S, Imokawa G. Signaling Cascades Activated by UVB in Human Melanocytes Lead to the Increased Expression of Melanocyte Receptors, Endothelin B Receptor and c-KIT. Photochem Photobiol. 2018;94:421-31
36. Nakajima H, Wakabayashi Y, Wakamatsu K, Imokawa G. An extract of Withania somnifera attenuates endothelin-1-stimulated pigmentation in human epidermal equivalents through the interruption of PKC activity within melanocytes. Phytother Res. 2011;25:1398-411
37. Hwang E, Lee TH, Lee W-J, Shim W-S, Yeo E-J, Kim S. et al. A novel syntheticPiperamide derivative NED-180 inhibits hyperpigmentation by activating the PI3K and ERK pathways and by regulating Ca2+influx via TRPM1 channels. Pigment Cell & Melanoma Research. 2016;29:81-91
38. Mosca S, Cardinali G, Flori E, Briganti S, Bottillo I, Mileo AM. et al. The PI3K pathway induced by alphaMSH exerts a negative feedback on melanogenesis and contributes to the release of pigment. Pigment Cell Melanoma Res. 2021;34:72-88
39. Jeon S, Kim NH, Kim JY, Lee AY. Stem cell factor induces ERM proteins phosphorylation through PI3K activation to mediate melanocyte proliferation and migration. Pigment Cell Melanoma Res. 2009;22:77-85
40. Todd JR, Scurr LL, Becker TM, Kefford RF, Rizos H. The MAPK pathway functions as a redundant survival signal that reinforces the PI3K cascade in c-Kit mutant melanoma. Oncogene. 2014;33:236-45
41. Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y. et al. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6:130-40
42. Murakami M, Matsuzaki F, Funaba M. Regulation of melanin synthesis by the TGF-beta family in B16 melanoma cells. Mol Biol Rep. 2009;36:1247-50
43. Klar AS, Biedermann T, Michalak K, Michalczyk T, Meuli-Simmen C, Scherberich A. et al. Human Adipose Mesenchymal Cells Inhibit Melanocyte Differentiation and the Pigmentation of Human Skin via Increased Expression of TGF-beta1. J Invest Dermatol. 2017;137:2560-9
44. Joompang A, Anwised P, Klaynongsruang S, Roytrakul S, Taemaitree L, Jangpromma N. Evaluation of TILI-2 as an Anti-Tyrosinase, Anti-Oxidative Agent and Its Role in Preventing Melanogenesis Using a Proteomics Approach. Molecules. 2022 27
45. Martinez-Esparza M, Jimenez-Cervantes C, Beermann F, Aparicio P, Lozano JA, Garcia-Borron JC. Transforming growth factor-beta1 inhibits basal melanogenesis in B16/F10 mouse melanoma cells by increasing the rate of degradation of tyrosinase and tyrosinase-related protein-1. J Biol Chem. 1997;272:3967-72
46. Kubic JD, Young KP, Plummer RS, Ludvik AE, Lang D. Pigmentation PAX-ways: the role of Pax3 in melanogenesis, melanocyte stem cell maintenance, and disease. Pigment Cell Melanoma Res. 2008;21:627-45
47. Yang G, Li Y, Nishimura EK, Xin H, Zhou A, Guo Y. et al. Inhibition of PAX3 by TGF-beta modulates melanocyte viability. Mol Cell. 2008;32:554-63
48. Kim DS, Park SH, Park KC. Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. Int J Biochem Cell Biol. 2004;36:1482-91
49. Moon HR, Jung JM, Kim SY, Song Y, Chang SE. TGF-beta3 suppresses melanogenesis in human melanocytes cocultured with UV-irradiated neighboring cells and human skin. J Dermatol Sci. 2020;99:100-8
50. Hibino T, Nishiyama T. Role of TGF-beta2 in the human hair cycle. J Dermatol Sci. 2004;35:9-18
51. Li S, Chen J, Chen F, Wang C, Guo X, Wang C. et al. Liposomal honokiol promotes hair growth via activating Wnt3a/beta-catenin signaling pathway and down regulating TGF-beta1 in C57BL/6N mice. Biomed Pharmacother. 2021;141:111793
52. Pierrat MJ, Marsaud V, Mauviel A, Javelaud D. Expression of microphthalmia-associated transcription factor (MITF), which is critical for melanoma progression, is inhibited by both transcription factor GLI2 and transforming growth factor-beta. J Biol Chem. 2012;287:17996-8004
53. Pierrat MJ, Marsaud V, Mauviel A, Javelaud D. Transcriptional repression of the tyrosinase-related protein 2 gene by transforming growth factor-beta and the Kruppel-like transcription factor GLI2. J Dermatol Sci. 2019;94:321-9
54. Steingrimsson E, Copeland NG, Jenkins NA. Melanocyte stem cell maintenance and hair graying. Cell. 2005;121:9-12
55. Gelmi MC, Houtzagers LE, Strub T, Krossa I, Jager MJ. MITF in Normal Melanocytes, Cutaneous and Uveal Melanoma: A Delicate Balance. Int J Mol Sci. 2022 23
56. Goding CR, Arnheiter H. MITF-the first 25 years. Genes Dev. 2019;33:983-1007
57. Du J, Widlund HR, Horstmann MA, Ramaswamy S, Ross K, Huber WE. et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 2004;6:565-76
58. Carreira S, Liu B, Goding CR. The gene encoding the T-box factor Tbx2 is a target for the microphthalmia-associated transcription factor in melanocytes. J Biol Chem. 2000;275:21920-7
59. Jacobs J, Keblusek P, Robanus-Maandag E, Kristel P, Lingbeek M, Nederlof P. et al. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19(ARF)) and is amplified in a subset of human breast cancers. Nature genetics. 2000;26:291-9
60. Prince S, Carreira S, Vance K, Abrahams A, Goding C. Tbx2 directly represses the expression of the p21(WAF1) cyclin-dependent kinase inhibitor. Cancer research. 2004;64:1669-74
61. Strub T, Giuliano S, Ye T, Bonet C, Keime C, Kobi D. et al. Essential role of microphthalmia transcription factor for DNA replication, mitosis and genomic stability in melanoma. Oncogene. 2011;30:2319-32
62. McGill G, Horstmann M, Widlund H, Du J, Motyckova G, Nishimura E. et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109:707-18
63. Dynek JN, Chan SM, Liu J, Zha J, Fairbrother WJ, Vucic D. Microphthalmia-associated transcription factor is a critical transcriptional regulator of melanoma inhibitor of apoptosis in melanomas. Cancer Res. 2008;68:3124-32
64. Beuret L, Flori E, Denoyelle C, Bille K, Busca R, Picardo M. et al. Up-regulation of MET expression by alpha-melanocyte-stimulating hormone and MITF allows hepatocyte growth factor to protect melanocytes and melanoma cells from apoptosis. J Biol Chem. 2007;282:14140-7
65. Levy C, Khaled M, Robinson KC, Veguilla RA, Chen PH, Yokoyama S. et al. Lineage-specific transcriptional regulation of DICER by MITF in melanocytes. Cell. 2010;141:994-1005
66. Zhang J, Mou Y, Gong H, Chen H, Xiao H. Microphthalmia-Associated Transcription Factor in Senescence and Age-Related Diseases. Gerontology. 2021;67:708-17
67. Seoane M, Buhs S, Iglesias P, Strauss J, Puller AC, Muller J. et al. Lineage-specific control of TFIIH by MITF determines transcriptional homeostasis and DNA repair. Oncogene. 2019;38:3616-35
68. Liu F, Fu Y, Meyskens FL Jr. MiTF regulates cellular response to reactive oxygen species through transcriptional regulation of APE-1/Ref-1. J Invest Dermatol. 2009;129:422-31
69. Buscà R, Berra E, Gaggioli C, Khaled M, Bille K, Marchetti B. et al. Hypoxia-inducible factor 1{alpha} is a new target of microphthalmia-associated transcription factor (MITF) in melanoma cells. The Journal of cell biology. 2005;170:49-59
70. Vazquez F, Lim JH, Chim H, Bhalla K, Girnun G, Pierce K. et al. PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell. 2013;23:287-301
71. Tanwar J, Sharma A, Saurav S, Shyamveer, Jatana N, Motiani R. MITF is a novel transcriptional regulator of the calcium sensor STIM1: Significance in physiological melanogenesis. The Journal of biological chemistry. 2022;298:102681
72. Chen J, Zheng Y, Hu C, Jin X, Chen X, Xiao Y. et al. Hair Graying Regulators Beyond Hair Follicle. Front Physiol. 2022;13:839859
73. Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552-65
74. Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V. et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5895-900
75. Zhang B, Ma S, Rachmin I, He M, Baral P, Choi S. et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature. 2020;577:676-81
76. Yuan XH, Jin ZH. Paracrine regulation of melanogenesis. Br J Dermatol. 2018;178:632-9
77. Moattari CR, Granstein RD. Neuropeptides and neurohormones in immune, inflammatory and cellular responses to ultraviolet radiation. Acta Physiol (Oxf). 2021;232:e13644
78. Samuelov L, Kinori M, Bertolini M, Paus R. Neural controls of human hair growth: calcitonin gene-related peptide (CGRP) induces catagen. J Dermatol Sci. 2012;67:153-5
79. Pi LQ, Jin XH, Hwang ST, Lee WS. Effects of calcitonin gene-related peptide on the immune privilege of human hair follicles. Neuropeptides. 2013;47:51-7
80. Toyoda M, Luo Y, Makino T, Matsui C, Morohashi M. Calcitonin gene-related peptide upregulates melanogenesis and enhances melanocyte dendricity via induction of keratinocyte-derived melanotrophic factors. J Investig Dermatol Symp Proc. 1999;4:116-25
81. Zhao J, Harada N, Kurihara H, Nakagata N, Okajima K. Dietary isoflavone increases insulin-like growth factor-I production, thereby promoting hair growth in mice. J Nutr Biochem. 2011;22:227-33
82. Zhou J, Feng JY, Wang Q, Shang J. Calcitonin gene-related peptide cooperates with substance P to inhibit melanogenesis and induces apoptosis of B16F10 cells. Cytokine. 2015;74:137-44
83. Liu N, Wang LH, Guo LL, Wang GQ, Zhou XP, Jiang Y. et al. Chronic restraint stress inhibits hair growth via substance P mediated by reactive oxygen species in mice. PLoS One. 2013;8:e61574
84. Peters EM, Liotiri S, Bodo E, Hagen E, Biro T, Arck PC. et al. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol. 2007;171:1872-86
85. Peters EM, Arck PC, Paus R. Hair growth inhibition by psychoemotional stress: a mouse model for neural mechanisms in hair growth control. Exp Dermatol. 2006;15:1-13
86. Paus R, Arck P, Tiede S. (Neuro-)endocrinology of epithelial hair follicle stem cells. Mol Cell Endocrinol. 2008;288:38-51
87. Ping F, Shang J, Zhou J, Song J, Zhang L. Activation of neurokinin-1 receptor by substance P inhibits melanogenesis in B16-F10 melanoma cells. Int J Biochem Cell Biol. 2012;44:2342-8
88. Zhou J, Geng KK, Ping FF, Gao YY, Liu L, Feng BN. Cross-talk between 5-hydroxytryptamine and substance P in the melanogensis and apoptosis of B16F10 melanoma cells. Eur J Pharmacol. 2016;775:106-12
89. Wu H, Zhao Y, Huang Q, Cai M, Pan Q, Fu M. et al. NK1R/5-HT1AR interaction is related to the regulation of melanogenesis. FASEB J. 2018;32:3193-214
90. Chen M, Cai J, Zhang X, Liao Z, Zhong M, Shang J. et al. Keratinocytes take part in the regulation of substance P in melanogenesis through the HPA axis. J Dermatol Sci. 2022;106:141-9
91. Park PJ, Lee TR, Cho EG. Substance P stimulates endothelin 1 secretion via endothelin-converting enzyme 1 and promotes melanogenesis in human melanocytes. J Invest Dermatol. 2015;135:551-9
92. Zhou J, Ling J, Song H, Lv B, Wang L, Shang J. et al. Neurokinin-1 receptor is a novel positive regulator of Wnt/ β-catenin signaling in melanogenesis. Oncotarget. 2016;7:81268-80
93. Bertolini M, Pretzlaff M, Sulk M, Bahr M, Gherardini J, Uchida Y. et al. Vasoactive intestinal peptide, whose receptor-mediated signalling may be defective in alopecia areata, provides protection from hair follicle immune privilege collapse. Br J Dermatol. 2016;175:531-41
94. Yuan XH, Yao C, Oh JH, Park CH, Tian YD, Han M. et al. Vasoactive intestinal peptide stimulates melanogenesis in B16F10 mouse melanoma cells via CREB/MITF/tyrosinase signaling. Biochem Biophys Res Commun. 2016;477:336-42
95. Guerrero-Juarez CF, Plikus MV. Emerging nonmetabolic functions of skin fat. Nat Rev Endocrinol. 2018;14:163-73
96. Kruglikov IL, Zhang Z, Scherer PE. The Role of Immature and Mature Adipocytes in Hair Cycling. Trends Endocrinol Metab. 2019;30:93-105
97. Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018;27:68-83
98. Nicu C, O'Sullivan JDB, Ramos R, Timperi L, Lai T, Farjo N. et al. Dermal Adipose Tissue Secretes HGF to Promote Human Hair Growth and Pigmentation. J Invest Dermatol. 2021;141:1633-45 e13
99. Chen J, Fan ZX, Zhu DC, Guo YL, Ye K, Dai D. et al. Emerging Role of Dermal White Adipose Tissue in Modulating Hair Follicle Development During Aging. Front Cell Dev Biol. 2021;9:728188
100. Won CH, Yoo HG, Park KY, Shin SH, Park WS, Park PJ. et al. Hair growth-promoting effects of adiponectin in vitro. J Invest Dermatol. 2012;132:2849-51
101. Park PJ, Cho EG. Kojyl Cinnamate Ester Derivatives Increase Adiponectin Expression and Stimulate Adiponectin-Induced Hair Growth Factors in Human Dermal Papilla Cells. Int J Mol Sci. 2019 20
102. Ohn J, Been KW, Kim JY, Kim EJ, Park T, Yoon HJ. et al. Discovery of a transdermally deliverable pentapeptide for activating AdipoR1 to promote hair growth. EMBO Mol Med. 2021;13:e13790
103. Nicu C, Jackson J, Shahmalak A, Pople J, Ansell D, Paus R. Adiponectin negatively regulates pigmentation, Wnt/β-catenin and HGF/c-Met signalling within human scalp hair follicles ex vivo. Arch Dermatol Res. 2023;315:603-12
104. Bang S, Won KH, Moon HR, Yoo H, Hong A, Song Y. et al. Novel regulation of melanogenesis by adiponectin via the AMPK/CRTC pathway. Pigment Cell Melanoma Res. 2017;30:553-7
105. Kim Y, Cho JY, Oh SW, Kang M, Lee SE, Jung E. et al. Globular adiponectin acts as a melanogenic signal in human epidermal melanocytes. Br J Dermatol. 2018;179:689-701
106. Xiong M, Zhang Q, Hu W, Zhao C, Lv W, Yi Y. et al. Exosomes From Adipose-Derived Stem Cells: The Emerging Roles and Applications in Tissue Regeneration of Plastic and Cosmetic Surgery. Front Cell Dev Biol. 2020;8:574223
107. Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int J Mol Sci. 2020 21
108. Tak YJ, Lee SY, Cho AR, Kim YS. A randomized, double-blind, vehicle-controlled clinical study of hair regeneration using adipose-derived stem cell constituent extract in androgenetic alopecia. Stem Cells Transl Med. 2020;9:839-49
109. Wu J, Yang Q, Wu S, Yuan R, Zhao X, Li Y. et al. Adipose-Derived Stem Cell Exosomes Promoted Hair Regeneration. Tissue Eng Regen Med. 2021;18:685-91
110. Nilforoushzadeh MA, Aghdami N, Taghiabadi E. Effects of Adipose-Derived Stem Cells and Platelet-Rich Plasma Exosomes on The Inductivity of Hair Dermal Papilla Cells. Cell J. 2021;23:576-83
111. Liang Y, Tang X, Zhang X, Cao C, Yu M, Wan M. Adipose Mesenchymal Stromal Cell-Derived Exosomes Carrying MiR-122-5p Antagonize the Inhibitory Effect of Dihydrotestosterone on Hair Follicles by Targeting the TGF-beta1/SMAD3 Signaling Pathway. Int J Mol Sci. 2023 24
112. Lu Q, Gao Y, Fan Z, Xiao X, Chen Y, Si Y. et al. Amphiregulin promotes hair regeneration of skin-derived precursors via the PI3K and MAPK pathways. Cell Prolif. 2021;54:e13106
113. Anderi R, Makdissy N, Azar A, Rizk F, Hamade A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res Ther. 2018;9:141
114. Kim DW, Jeon BJ, Hwang NH, Kim MS, Park SH, Dhong ES. et al. Adipose-derived stem cells inhibit epidermal melanocytes through an interleukin-6-mediated mechanism. Plast Reconstr Surg. 2014;134:470-80
115. Chang H, Park JH, Min KH, Lee RS, Kim EK. Whitening effects of adipose-derived stem cells: a preliminary in vivo study. Aesthetic Plast Surg. 2014;38:230-3
116. Jeon BJ, Kim DW, Kim MS, Park SH, Dhong ES, Yoon ES. et al. Protective effects of adipose-derived stem cells against UVB-induced skin pigmentation. J Plast Surg Hand Surg. 2016;50:336-42
117. Dou S, Yang Y, Zhang J, He Z, Wu Z, Zhao Y. et al. Exploring the Role and Mechanism of Adipose Derived Mesenchymal Stem Cells on Reversal of Pigmentation Model Effects. Aesthetic Plast Surg. 2022;46:1983-96
118. Shen JP, Wu YX, Tang SJ, Peng LH. Experimental study on stromal vascular fraction mediated inhibition of skin pigmentation in guinea pigs. Ann Transl Med. 2022;10:1268
119. Kim JY, Park CD, Lee JH, Lee CH, Do BR, Lee AY. Co-culture of melanocytes with adipose-derived stem cells as a potential substitute for co-culture with keratinocytes. Acta Derm Venereol. 2012;92:16-23
120. Lim WS, Kim CH, Kim JY, Do BR, Kim EJ, Lee AY. Adipose-derived stem cells improve efficacy of melanocyte transplantation in animal skin. Biomol Ther (Seoul). 2014;22:328-33
121. Kim H, Yi N, Do BR, Lee AY. Adipose-Derived Stem Cell Coculturing Stimulates Integrin-Mediated Extracellular Matrix Adhesion of Melanocytes by Upregulating Growth Factors. Biomol Ther (Seoul). 2019;27:185-92
122. Bellei B, Papaccio F, Filoni A, Caputo S, Lopez G, Migliano E. et al. Extracellular fraction of adipose tissue as an innovative regenerative approach for vitiligo treatment. Exp Dermatol. 2019;28:695-703
123. Bian Y, Yu H, Jin M, Gao X. Repigmentation by combined narrow-band ultraviolet B/adipose-derived stem cell transplantation in the mouse model: Role of Nrf2/HO-1-mediated Ca(2+) homeostasis. Mol Med Rep. 2022 25
124. Castelli MS, McGonigle P, Hornby PJ. The pharmacology and therapeutic applications of monoclonal antibodies. Pharmacol Res Perspect. 2019;7:e00535
125. Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother. 2019;15:1111-22
126. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21:28
127. Rivera N, Boada A, Bielsa MI, Fernandez-Figueras MT, Carcereny E, Moran MT. et al. Hair Repigmentation During Immunotherapy Treatment With an Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Agent for Lung Cancer. JAMA Dermatol. 2017;153:1162-5
128. Manson G, Marabelle A, Houot R. Hair Repigmentation With Anti-PD-1 and Anti-PD-L1 Immunotherapy: A Novel Hypothesis. JAMA Dermatol. 2018;154:113
129. Sebaratnam DF, Rodriguez Bandera AI, Lowe PM. Hair Repigmentation With Anti-PD-1 and Anti-PD-L1 Immunotherapy: A Novel Hypothesis. JAMA Dermatol. 2018;154:112-3
130. Park M, Woo SY, Cho KA, Cho MS, Lee KH. PD-L1 produced by HaCaT cells under polyinosinic-polycytidylic acid stimulation inhibits melanin production by B16F10 cells. PLoS One. 2020;15:e0233448
131. Quach HT, Johnson DB, LeBoeuf NR, Zwerner JP, Dewan AK. Cutaneous adverse events caused by immune checkpoint inhibitors. J Am Acad Dermatol. 2021;85:956-66
132. Correa-Selm LM, Grichnik JM. PD1 inhibitors and hair repigmentation: A desirable new side effect. Dermatol Ther. 2018 31
133. Navarro-Trivino FJ, Ruiz-Villaverde R, Manuel Ramos-Pleguezuelos F, Vano-Galvan S. Canities Subita after Extreme Trauma Showing Positive Staining for Anti-PD-L1 Antibodies: A New Clue into Etiopathogenesis?. Skin Appendage Disord. 2022;8:65-9
134. Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy. 2020;50:5-14
135. Sumitomo C, Akita H, Sugiura K. Unexpected side-effect of dupilumab: Reversal of hair graying. J Dermatol. 2020;47:e316-e7
136. Choi H, Choi H, Han J, Jin SH, Park JY, Shin DW. et al. IL-4 Inhibits the Melanogenesis of Normal Human Melanocytes through the JAK2-STAT6 Signaling Pathway. J Invest Dermatol. 2013;133:528-36
137. Tintle S, Dabade T, Kalish R, Rosmarin D. Repigmentation of hair following adalimumab therapy. Dermatology online journal. 2015 21
138. Singh M, Mansuri MS, Kadam A, Palit SP, Dwivedi M, Laddha NC. et al. Tumor Necrosis Factor-alpha affects melanocyte survival and melanin synthesis via multiple pathways in vitiligo. Cytokine. 2021;140:155432
139. Wang CQF, Akalu YT, Suarez-Farinas M, Gonzalez J, Mitsui H, Lowes MA. et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-52
140. Grine L, Dejager L, Libert C, Vandenbroucke RE. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015;26:25-33
141. Martínez-Esparza M, Jiménez-Cervantes C, Solano F, Lozano J, García-Borrón J. Regulation of the murine silver locus product (gp87) by the hypopigmenting cytokines TGF-beta1 and TNF-alpha. Pigment cell research. 2000;13:120-6
142. Funasaka Y, Chakraborty A, Hayashi Y, Komoto M, Ohashi A, Nagahama M. et al. Modulation of melanocyte-stimulating hormone receptor expression on normal human melanocytes: evidence for a regulatory role of ultraviolet B, interleukin-1alpha, interleukin-1beta, endothelin-1 and tumour necrosis factor-alpha. The British journal of dermatology. 1998;139:216-24
143. Choi H, Ahn S, Lee BG, Chang I, Hwang JS. Inhibition of skin pigmentation by an extract of Lepidium apetalum and its possible implication in IL-6 mediated signaling. Pigment Cell Res. 2005;18:439-46
144. Choi H, Kim K, Han J, Choi H, Jin SH, Lee EK. et al. Kojic acid-induced IL-6 production in human keratinocytes plays a role in its anti-melanogenic activity in skin. J Dermatol Sci. 2012;66:207-15
145. Jiang L, Huang J, Lu J, Hu S, Pei S, Ouyang Y. et al. Ganoderma lucidum polysaccharide reduces melanogenesis by inhibiting the paracrine effects of keratinocytes and fibroblasts via IL-6/STAT3/FGF2 pathway. J Cell Physiol. 2019;234:22799-808
146. Camara-Lemarroy CR, Salas-Alanis JC. The role of tumor necrosis factor-alpha in the pathogenesis of vitiligo. Am J Clin Dermatol. 2013;14:343-50
147. Manaka L, Kadono S, Kawashima M, Kobayashi T, Imokawa G. The mechanism of hyperpigmentation in seborrhoeic keratosis involves the high expression of endothelin-converting enzyme-1alpha and TNF-alpha, which stimulate secretion of endothelin 1. The British journal of dermatology. 2001;145:895-903
148. Imokawa G. Melanocyte Activation Mechanisms and Rational Therapeutic Treatments of Solar Lentigos. Int J Mol Sci. 2019 20
149. Takenaka Y, Hoshino Y, Nakajima H, Hayashi N, Kawashima M, Imokawa G. Paracrine cytokine mechanisms underlying the hyperpigmentation of seborrheic keratosis in covered skin areas. J Dermatol. 2013;40:533-42
150. Lu Y, Tonissen KF, Di Trapani G. Modulating skin colour: role of the thioredoxin and glutathione systems in regulating melanogenesis. Biosci Rep. 2021 41
151. Rongioletti F, Mugheddu C, Murgia S. Repigmentation and new growth of hairs after anti-interleukin-17 therapy with secukinumab for psoriasis. JAAD Case Rep. 2018;4:486-8
152. Zhou J, An X, Dong J, Wang Y, Zhong H, Duan L. et al. IL-17 induces cellular stress microenvironment of melanocytes to promote autophagic cell apoptosis in vitiligo. FASEB J. 2018;32:4899-916
153. Kotobuki Y, Tanemura A, Yang L, Itoi S, Wataya-Kaneda M, Murota H. et al. Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res. 2012;25:219-30
154. Kholmanskikh O, van Baren N, Brasseur F, Ottaviani S, Vanacker J, Arts N. et al. Interleukins 1alpha and 1beta secreted by some melanoma cell lines strongly reduce expression of MITF-M and melanocyte differentiation antigens. Int J Cancer. 2010;127:1625-36
155. Arts N, Cane S, Hennequart M, Lamy J, Bommer G, Van den Eynde B. et al. microRNA-155, induced by interleukin-1ss, represses the expression of microphthalmia-associated transcription factor (MITF-M) in melanoma cells. PLoS One. 2015;10:e0122517
156. Zhuang T, Li S, Yi X, Guo S, Wang Y, Chen J. et al. Tranilast Directly Targets NLRP3 to Protect Melanocytes From Keratinocyte-Derived IL-1beta Under Oxidative Stress. Front Cell Dev Biol. 2020;8:588
157. Yang CY, Guo Y, Wu WJ, Man MQ, Tu Y, He L. UVB-Induced Secretion of IL-1beta Promotes Melanogenesis by Upregulating TYR/TRP-1 Expression In vitro. Biomed Res Int. 2022;2022:8230646
158. Singh RK, Lee KM, Vujkovic-Cvijin I, Ucmak D, Farahnik B, Abrouk M. et al. The role of IL-17 in vitiligo: A review. Autoimmun Rev. 2016;15:397-404
159. Bernardini N, Skroza N, Tolino E, Mambrin A, Anzalone A, Balduzzi V. et al. IL-17 and its role in inflammatory, autoimmune, and oncological skin diseases: state of art. Int J Dermatol. 2020;59:406-11
160. Bhardwaj S, Rani S, Srivastava N, Kumar R, Parsad D. Increased systemic and epidermal levels of IL-17A and IL-1beta promotes progression of non-segmental vitiligo. Cytokine. 2017;91:153-61
161. Tomaszewska K, Kozlowska M, Kaszuba A, Lesiak A, Narbutt J, Zalewska-Janowska A. Increased Serum Levels of IFN-gamma, IL-1beta, and IL-6 in Patients with Alopecia Areata and Nonsegmental Vitiligo. Oxid Med Cell Longev. 2020;2020:5693572
162. Di Cesare A, Fargnoli MC, Marinucci A, Peris K. Rationale for the development of speckled hyperpigmentation in the areas of psoriatic plaques after treatment with biologic agents. J Invest Dermatol. 2015;135:318-20
163. Zhang S, Liang J, Tian X, Zhou X, Liu W, Chen X. et al. Secukinumab-induced multiple lentigines in areas of resolved psoriatic plaques: A case report and literature review. Dermatol Ther. 2021;34:e15048
164. Park S, Ahn G, Park J, Seo S. The First Case of Ustekinumab-Associated Hair Repigmentation and a Proposed Mechanism of Action. Annals of dermatology. 2021;33:300-1
165. Armstrong AW, Read C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA. 2020;323:1945-60
166. Penzi LR, Manatis-Lornell A, Saavedra A, Fisher D, Senna MM. Hair repigmentation associated with the use of brentuximab. JAAD Case Rep. 2017;3:563-5
167. Deutsch YE, Tadmor T, Podack ER, Rosenblatt JD. CD30: an important new target in hematologic malignancies. Leuk Lymphoma. 2011;52:1641-54
168. So T, Ishii N. The TNF-TNFR Family of Co-signal Molecules. Advances in experimental medicine and biology. 2019;1189:53-84
169. Robert C, Spatz A, Faivre S, Armand JP, Raymond E. Tyrosine kinase inhibition and grey hair. Lancet. 2003;361:1056
170. Kockerols C, Westerweel P. Hair Repigmentation Induced by Nilotinib. The New England journal of medicine. 2022;387:e12
171. Cheng Y, Chen H, Chiu H. Erlotinib-induced hair repigmentation. International journal of dermatology. 2014;53:e55-7
172. Robert C, Mateus C, Spatz A, Wechsler J, Escudier B. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol. 2009;60:299-305
173. Cohen P, Cross D, Janne PA. Kinase drug discovery 20 years after imatinib: progress and future directions. Nat Rev Drug Discov. 2021;20:551-69
174. Quintás-Cardama A, Cortes J. Nilotinib: a phenylamino-pyrimidine derivative with activity against BCR-ABL, KIT and PDGFR kinases. Future oncology (London, England). 2008;4:611-21
175. Contreras O, Villarreal M, Brandan E. Nilotinib impairs skeletal myogenesis by increasing myoblast proliferation. Skelet Muscle. 2018;8:5
176. Kim J, Lee HJ, Park JH, Cha BY, Hoe HS. Nilotinib modulates LPS-induced cognitive impairment and neuroinflammatory responses by regulating P38/STAT3 signaling. J Neuroinflammation. 2022;19:187
177. Jeitany M, Leroy C, Tosti P, Lafitte M, Le Guet J, Simon V. et al. Inhibition of DDR1-BCR signalling by nilotinib as a new therapeutic strategy for metastatic colorectal cancer. EMBO Mol Med. 2018 10
178. Wu J, Xu X, Zheng L, Mo J, Jin X, Bao Y. Nilotinib inhibits microglia-mediated neuroinflammation to protect against dopaminergic neuronal death in Parkinson's disease models. Int Immunopharmacol. 2021;99:108025
179. Kim KI, Jo JW, Lee JH, Kim CD, Yoon TJ. Induction of pigmentation by a small molecule tyrosine kinase inhibitor nilotinib. Biochem Biophys Res Commun. 2018;503:2271-6
180. Chang SP, Huang HM, Shen SC, Lee WR, Chen YC. Nilotinib induction of melanogenesis via reactive oxygen species-dependent JNK activation in B16F0 mouse melanoma cells. Exp Dermatol. 2018;27:1388-94
181. Kang B, Kim Y, Park TJ, Kang HY. Dasatinib, a second-generation tyrosine kinase inhibitor, induces melanogenesis via ERK-CREB-MITF-tyrosinase signaling in normal human melanocytes. Biochem Biophys Res Commun. 2020;523:1034-9
182. Jeong SM, Yoon TJ. Development of Pigmentation-Regulating Agents by Drug Repositioning. Int J Mol Sci. 2021 22
183. Kim KI, Jung KE, Shin YB, Kim CD, Yoon TJ. Sorafenib induces pigmentation via the regulation of beta-catenin signalling pathway in melanoma cells. Exp Dermatol. 2022;31:57-63
184. Steins M, Thomas M, Geissler M. Erlotinib. Recent Results Cancer Res. 2018;211:1-17
185. Lin KY, Chen CM, Lu CY, Cheng CY, Wu YH. Regulation of miR-21 expression in human melanoma via UV-ray-induced melanin pigmentation. Environ Toxicol. 2017;32:2064-9
186. Yun WJ, Bang SH, Min KH, Kim SW, Lee MW, Chang SE. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013;39:1903-11
187. AlGhamdi KM, Kumar A. Depigmentation therapies for normal skin in vitiligo universalis. J Eur Acad Dermatol Venereol. 2011;25:749-57
188. Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-18 quiz 19-20
189. Zuo RC, Apolo AB, DiGiovanna JJ, Parnes HL, Keen CM, Nanda S. et al. Cutaneous adverse effects associated with the tyrosine-kinase inhibitor cabozantinib. JAMA Dermatol. 2015;151:170-7
190. Lovering S, Miao W, Bailie T, Amato D. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016. 2016
191. Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process!. J Oncol Pharm Pract. 2013;19:165-9
192. Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma. 2013;54:683-7
193. Chang X, Zhu Y, Shi C, Stewart AK. Mechanism of immunomodulatory drugs' action in the treatment of multiple myeloma. Acta Biochim Biophys Sin (Shanghai). 2014;46:240-53
194. Xu Y, Sun J, Sheard MA, Tran HC, Wan Z, Liu WY. et al. Lenalidomide overcomes suppression of human natural killer cell anti-tumor functions by neuroblastoma microenvironment-associated IL-6 and TGFbeta1. Cancer Immunol Immunother. 2013;62:1637-48
195. Liang CJ, Yen YH, Hung LY, Wang SH, Pu CM, Chien HF. et al. Thalidomide inhibits fibronectin production in TGF-beta1-treated normal and keloid fibroblasts via inhibition of the p38/Smad3 pathway. Biochem Pharmacol. 2013;85:1594-602
196. Bian C, Qin WJ, Zhang CY, Zou GL, Zhu YZ, Chen J. et al. Thalidomide (THD) alleviates radiation induced lung fibrosis (RILF) via down-regulation of TGF-beta/Smad3 signaling pathway in an Nrf2-dependent manner. Free Radic Biol Med. 2018;129:446-53
197. Lu Y, Zhao C, Lei L, Tao Z, Zheng L, Wen J. et al. Effects of thalidomide on Th17, Treg cells and TGF-beta1/Smad3 pathway in a mouse model of systemic sclerosis. Int J Rheum Dis. 2020;23:406-19
198. Amirshahrokhi K, Khalili AR. Thalidomide ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in an experimental model. Inflammation. 2015;38:476-84
199. Paravar T, Lee DJ. Thalidomide: mechanisms of action. Int Rev Immunol. 2008;27:111-35
200. Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS Jr. Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem. 2001;276:22382-7
201. Fu C, Chen J, Lu J, Yi L, Tong X, Kang L. et al. Roles of inflammation factors in melanogenesis (Review). Mol Med Rep. 2020;21:1421-30
202. Zhou J, Ling J, Song J, Wang Y, Feng B, Ping F. Interleukin 10 protects primary melanocyte by activation of Stat-3 and PI3K/Akt/NF-kappaB signaling pathways. Cytokine. 2016;83:275-81
203. Semeraro M, Vacchelli E, Eggermont A, Galon J, Zitvogel L, Kroemer G. et al. Trial Watch: Lenalidomide-based immunochemotherapy. Oncoimmunology. 2013;2:e26494
204. Englaro W, Bahadoran P, Bertolotto C, Buscà R, Dérijard B, Livolsi A. et al. Tumor necrosis factor alpha-mediated inhibition of melanogenesis is dependent on nuclear factor kappa B activation. Oncogene. 1999;18:1553-9
205. Kim K, Choi H, Kim H, Lee T. TNFSF14 inhibits melanogenesis via NF-kB signaling in melanocytes. Cytokine. 2018;110:126-30
206. Zhou J, Shang J, Song J, Ping F. Interleukin-18 augments growth ability of primary human melanocytes by PTEN inactivation through the AKT/NF-kappaB pathway. Int J Biochem Cell Biol. 2013;45:308-16
207. Sun L, Pan S, Yang Y, Sun J, Liang D, Wang X. et al. Toll-like receptor 9 regulates melanogenesis through NF-kappaB activation. Exp Biol Med (Maywood). 2016;241:1497-504
208. Chaiprasongsuk A, Panich U. Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Front Pharmacol. 2022;13:823881
209. Bodera P, Stankiewicz W. Immunomodulatory properties of thalidomide analogs: pomalidomide and lenalidomide, experimental and therapeutic applications. Recent patents on endocrine, metabolic & immune drug discovery. 2011;5:192-6
210. Wang Y, Viennet C, Robin S, Berthon JY, He L, Humbert P. Precise role of dermal fibroblasts on melanocyte pigmentation. J Dermatol Sci. 2017;88:159-66
211. Kim NH, Lee AY. Growth Factors Upregulated by Uric Acid Affect Guanine Deaminase-Induced Melanogenesis. Biomol Ther (Seoul). 2023;31:89-96
212. Zhu JW, Ni YJ, Tong XY, Guo X, Wu XP. Activation of VEGF receptors in response to UVB promotes cell proliferation and melanogenesis of normal human melanocytes. Exp Cell Res. 2020;387:111798
213. Zhu JW, Ni YJ, Tong XY, Guo X, Wu XP, Lu ZF. Tranexamic Acid Inhibits Angiogenesis and Melanogenesis in vitro by Targeting VEGF Receptors. Int J Med Sci. 2020;17:903-11
214. Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63:925-46 quiz 47-8
215. Rebora A, Delmonte S, Parodi A. Cyclosporin A-induced hair darkening. International journal of dermatology. 1999;38:229-30
216. Sadighha A, Zahed GM. Hair darkening after treatment with cyclosporin in a patient with psoriasis. J Eur Acad Dermatol Venereol. 2008;22:1239-41
217. Gohar A. Comment on the letter by Sadighha and Zahed on Hair darkening after treatment with cyclosporin in a patient with psoriasis. Journal of the European Academy of Dermatology and Venereology: JEADV. 2009;23:862
218. Hawkshaw NJ, Paus R. Beyond the NFAT Horizon: From Cyclosporine A-Induced Adverse Skin Effects to Novel Therapeutics. Trends Pharmacol Sci. 2021;42:316-28
219. Hawkshaw NJ, Hardman JA, Haslam IS, Shahmalak A, Gilhar A, Lim X. et al. Identifying novel strategies for treating human hair loss disorders: Cyclosporine A suppresses the Wnt inhibitor, SFRP1, in the dermal papilla of human scalp hair follicles. PLoS Biol. 2018;16:e2003705
220. Redondo P, Guzmán M, Marquina M, Pretel M, Aguado L, Lloret P. et al. [Repigmentation of gray hair after thyroid hormone treatment]. Actas dermo-sifiliograficas. 2007;98:603-10
221. van Beek N, Bodo E, Kromminga A, Gaspar E, Meyer K, Zmijewski MA. et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-8
222. Hardman JA, Haslam IS, Farjo N, Farjo B, Paus R. Thyroxine differentially modulates the peripheral clock: lessons from the human hair follicle. PLoS One. 2015;10:e0121878
223. Mancino G, Miro C, Di Cicco E, Dentice M. Thyroid hormone action in epidermal development and homeostasis and its implications in the pathophysiology of the skin. J Endocrinol Invest. 2021;44:1571-9
224. Di Cicco E, Moran C, Visser WE, Nappi A, Schoenmakers E, Todd P. et al. Germ Line Mutations in the Thyroid Hormone Receptor Alpha Gene Predispose to Cutaneous Tags and Melanocytic Nevi. Thyroid. 2021;31:1114-26
225. Bellandi S, Amato L, Cipollini E, Antiga E, Brandini L, Fabbri P. Repigmentation of hair after latanoprost therapy. Journal of the European Academy of Dermatology and Venereology: JEADV. 2011;25:1485-7
226. Digiuni M, Fogagnolo P, Rossetti L. A review of the use of latanoprost for glaucoma since its launch. Expert opinion on pharmacotherapy. 2012;13:723-45
227. Scott G, Leopardi S, Printup S, Malhi N, Seiberg M, Lapoint R. Proteinase-activated receptor-2 stimulates prostaglandin production in keratinocytes: analysis of prostaglandin receptors on human melanocytes and effects of PGE2 and PGF2alpha on melanocyte dendricity. The Journal of investigative dermatology. 2004;122:1214-24
228. Scott G, Jacobs S, Leopardi S, Anthony FA, Learn D, Malaviya R. et al. Effects of PGF2alpha on human melanocytes and regulation of the FP receptor by ultraviolet radiation. Exp Cell Res. 2005;304:407-16
229. Gledhill K, Rhodes LE, Brownrigg M, Haylett AK, Masoodi M, Thody AJ. et al. Prostaglandin-E2 is produced by adult human epidermal melanocytes in response to UVB in a melanogenesis-independent manner. Pigment Cell Melanoma Res. 2010;23:394-403
230. Starner RJ, McClelland L, Abdel-Malek Z, Fricke A, Scott G. PGE(2) is a UVR-inducible autocrine factor for human melanocytes that stimulates tyrosinase activation. Exp Dermatol. 2010;19:682-4
231. Ma HJ, Ma HY, Yang Y, Li PC, Zi SX, Jia CY. et al. alpha-Melanocyte stimulating hormone (MSH) and prostaglandin E2 (PGE2) drive melanosome transfer by promoting filopodia delivery and shedding spheroid granules: Evidences from atomic force microscopy observation. J Dermatol Sci. 2014;76:222-30
232. Shin DW. The physiological and pharmacological roles of prostaglandins in hair growth. Korean J Physiol Pharmacol. 2022;26:405-13
233. Yazdanian N, Mozafarpoor S, Goodarzi A. Phosphodiesterase inhibitors and prostaglandin analogues in dermatology: A comprehensive review. Dermatol Ther. 2021;34:e14669
234. Sasaki S, Hozumi Y, Kondo S. Influence of prostaglandin F2alpha and its analogues on hair regrowth and follicular melanogenesis in a murine model. Experimental dermatology. 2005;14:323-8
235. Chan LKM, Braidy N, Ng W, Xu YH, Chen J, McDonald R. et al. Re-pigmentation of hair after prolonged cholinesterase inhibitor therapy in a Chinese population. Australas J Dermatol. 2020;61:e417-e20
236. Wu Q, Xia Y, Dai K, Bai P, Kwan KKL, Guo MSS. et al. Solar light induces the release of acetylcholine from skin keratinocytes affecting melanogenesis. FASEB J. 2020;34:8941-58
237. Wu Q, Fung AHY, Xu ML, Poon K, Liu EYL, Kong XP. et al. Microphthalmia-associated transcription factor up-regulates acetylcholinesterase expression during melanogenesis of murine melanoma cells. J Biol Chem. 2018;293:14417-28
238. Wu Q, Bai P, Xia Y, Lai QWS, Guo MSS, Dai K. et al. Solar light induces expression of acetylcholinesterase in skin keratinocytes: Signalling mediated by activator protein 1 transcription factor. Neurochem Int. 2020;141:104861
239. Guo M, Wu Q, Dong T, Tsim K. The UV-induced uptake of melanosome by skin keratinocyte is triggered by α7 nicotinic acetylcholine receptor-mediated phagocytosis. The FEBS journal. 2022
240. Hasse S, Chernyavsky AI, Grando SA, Paus R. The M4 muscarinic acetylcholine receptor plays a key role in the control of murine hair follicle cycling and pigmentation. Life Sci. 2007;80:2248-52
241. Enkhtaivan E, Lee CH. Role of Amine Neurotransmitters and Their Receptors in Skin Pigmentation: Therapeutic Implication. Int J Mol Sci. 2021 22
242. Takahashi T. Multiple Roles for Cholinergic Signaling from the Perspective of Stem Cell Function. Int J Mol Sci. 2021 22
243. Hampson J, Donnelly A, Lewis-Jones M, Pye J. Tamoxifen-induced hair colour change. The British journal of dermatology. 1995;132:483-4
244. Cario M. How hormones may modulate human skin pigmentation in melasma: An in vitro perspective. Exp Dermatol. 2019;28:709-18
245. Jian D, Jiang D, Su J, Chen W, Hu X, Kuang Y. et al. Diethylstilbestrol enhances melanogenesis via cAMP-PKA-mediating up-regulation of tyrosinase and MITF in mouse B16 melanoma cells. Steroids. 2011;76:1297-304
246. Sun M, Xie HF, Tang Y, Lin SQ, Li JM, Sun SN. et al. G protein-coupled estrogen receptor enhances melanogenesis via cAMP-protein kinase (PKA) by upregulating microphthalmia-related transcription factor-tyrosinase in melanoma. J Steroid Biochem Mol Biol. 2017;165:236-46
247. Filoni A, Mariano M, Cameli N. Melasma: How hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-63
248. Matama T, Araujo R, Preto A, Cavaco-Paulo A, Gomes AC. In vitro induction of melanin synthesis and extrusion by tamoxifen. Int J Cosmet Sci. 2013;35:368-74
249. Yang G, Nowsheen S, Aziz K, Georgakilas AG. Toxicity and adverse effects of Tamoxifen and other anti-estrogen drugs. Pharmacol Ther. 2013;139:392-404
250. Komagamine T, Suzuki K, Hirata K. Darkening of white hair following levodopa therapy in a patient with Parkinson's disease. Mov Disord. 2013;28:1643
251. Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25:14-27
252. Langan EA, Lisztes E, Biro T, Funk W, Kloepper JE, Griffiths CE. et al. Dopamine is a novel, direct inducer of catagen in human scalp hair follicles in vitro. Br J Dermatol. 2013;168:520-5
253. Villarreal-Reyna G, Garza-Morales R, Soto-Dominguez A, Montanez-Guerrero L, Saucedo-Cardenas O, Gomez-Flores M. et al. Cerebrolysin induces hair repigmentation associated to MART-1/Melan-A reactivation. Eur J Med Res. 2022;27:257
254. Botchkarev V, Botchkareva N, Albers K, Chen L, Welker P, Paus R. A role for p75 neurotrophin receptor in the control of apoptosis-driven hair follicle regression. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2000;14:1931-42
255. Nagase K, Inoue T, Narisawa Y. Manifest hair repigmentation associated with etretinate therapy. J Dermatol. 2017;44:e34-e5
256. Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol. 2009;50:214-6
257. Ward PD, Miller HL, Shipman AR. A case of repigmentation and curling of hair on acitretin therapy. Clin Exp Dermatol. 2014;39:91-2
258. Vesper J, Fenske N. Hair darkening and new growth associated with etretinate therapy. Journal of the American Academy of Dermatology. 1996;34:860
259. VanBuren CA, Everts HB. Vitamin A in Skin and Hair: An Update. Nutrients. 2022 14
260. Wang Z, Coleman DJ, Bajaj G, Liang X, Ganguli-Indra G, Indra AK. RXRalpha ablation in epidermal keratinocytes enhances UVR-induced DNA damage, apoptosis, and proliferation of keratinocytes and melanocytes. J Invest Dermatol. 2011;131:177-87
261. Taguchi N, Hata T, Kamiya E, Homma T, Kobayashi A, Aoki H. et al. Eriodictyon angustifolium extract, but not Eriodictyon californicum extract, reduces human hair greying. Int J Cosmet Sci. 2020;42:336-45
262. Taguchi N, Homma T, Aoki H, Kunisada T. Dietary Eriodictyon angustifolium Tea Supports Prevention of Hair Graying by Reducing DNA Damage in CD34+ Hair Follicular Keratinocyte Stem Cells. Biological & pharmaceutical bulletin. 2020;43:1451-4
263. Taguchi N, Hata T, Kamiya E, Kobayashi A, Aoki H, Kunisada T. Reduction in human hair graying by sterubin, an active flavonoid of Eriodictyon angustifolium. J Dermatol Sci. 2018;92:286-9
264. Taguchi N, Kitai R, Ando T, Nishimura T, Aoki H, Kunisada T. Protective Effect of Hydroxygenkwanin against Hair Graying Induced by X-Ray Irradiation and Repetitive Plucking. JID Innov. 2022;2:100121
265. Han MN, Lu JM, Zhang GY, Yu J, Zhao RH. Mechanistic Studies on the Use of Polygonum multiflorum for the Treatment of Hair Graying. Biomed Res Int. 2015;2015:651048
266. Sextius P, Betts R, Benkhalifa I, Commo S, Eilstein J, Massironi M. et al. Polygonum multiflorum Radix extract protects human foreskin melanocytes from oxidative stress in vitro and potentiates hair follicle pigmentation ex vivo. International journal of cosmetic science. 2017;39:419-25
267. Thang ND, Diep PN, Lien PT, Lien LT. Polygonum multiflorum root extract as a potential candidate for treatment of early graying hair. J Adv Pharm Technol Res. 2017;8:8-13
268. Jo SJ, Shin H, Paik SH, Na SJ, Jin Y, Park WS. et al. Efficacy and Safety of Pueraria lobata Extract in Gray Hair Prevention: A Randomized, Double-Blind, Placebo-Controlled Study. Ann Dermatol. 2013;25:218-22
269. Park WS, Kwon O, Yoon TJ, Chung JH. Anti-graying effect of the extract of Pueraria thunbergiana via upregulation of cAMP/MITF-M signaling pathway. J Dermatol Sci. 2014;75:153-5
270. Chavez A, Tiger J. Hair Repigmentation After Mohs Micrographic Surgery and Secondary Intention Wound Healing on the Scalp of an 84-Year-Old Woman. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al]. 2021;47:1281-3
271. Kubelis-Lopez DE, Zapata-Salazar NA, Said-Fernandez SL, Sanchez-Dominguez CN, Salinas-Santander MA, Martinez-Rodriguez HG. et al. Updates and new medical treatments for vitiligo (Review). Exp Ther Med. 2021;22:797
272. Ziaeifar E, Ziaeifar F, Mozafarpoor S, Goodarzi A. Applications of microneedling for various dermatologic indications with a special focus on pigmentary disorders: A comprehensive review study. Dermatologic therapy. 2021;34:e15159
273. York K, Meah N, Bhoyrul B, Sinclair R. A review of the treatment of male pattern hair loss. Expert Opin Pharmacother. 2020;21:603-12
274. Yuriguchi M, Aoki H, Taguchi N, Kunisada T. Pigmentation of regenerated hairs after wounding. Journal of Dermatological Science. 2016;84:80-7
275. Goldstein NB, Koster MI, Jones KL, Gao B, Hoaglin LG, Robinson SE. et al. Repigmentation of Human Vitiligo Skin by NBUVB Is Controlled by Transcription of GLI1 and Activation of the beta-Catenin Pathway in the Hair Follicle Bulge Stem Cells. J Invest Dermatol. 2018;138:657-68
276. Han X, Chang L, Qiu Z, Lin M, Wang Y, Liu D. et al. Micro-Injury Induces Hair Regeneration and Vitiligo Repigmentation Through Wnt/beta-Catenin Pathway. Stem Cells Dev. 2022;31:111-8
277. Li H, Fan L, Zhu S, Shin MK, Lu F, Qu J. et al. Epilation induces hair and skin pigmentation through an EDN3/EDNRB-dependent regenerative response of melanocyte stem cells. Sci Rep. 2017;7:7272
278. Dummer R. Clinical picture: hair repigmentation in lentigo maligna. Lancet. 2001;357:598
279. Rahim RR, Husain A, Tobin DJ, Lawrence CM. Desmoplastic melanoma presenting with localized hair repigmentation. Br J Dermatol. 2013;169:1371-3
280. Inzinger M, Massone C, Arzberger E, Hofmann-Wellenhof R. Hair repigmentation in melanoma. Lancet. 2013;382:1224
281. Tiger JB, Habeshian KA, Barton DT, Brennick JB. Repigmentation of hair associated with melanoma in situ of scalp. J Am Acad Dermatol. 2014;71:e144-5
282. Amann VC, Dummer R. Localized Hair Repigmentation in a 91-Year-Old Woman. JAMA Dermatol. 2016;152:81-2
283. Chan C, Magro C, Pham A, LeBlanc R, Yan S, Barton D. et al. Spontaneous Hair Repigmentation in an 80-Year-Old Man: A Case of Melanoma-Associated Hair Repigmentation and Review of the Literature. The American Journal of dermatopathology. 2019;41:671-4
284. Lackey AE, Glassman G, Grichnik J, McDonald J, Correa-Selm L. Repigmentation of gray hairs with lentigo maligna and response to topical imiquimod. JAAD Case Rep. 2019;5:1015-7
285. Lopez-Sanchez C, Collgros H. Hair repigmentation as a clue for scalp melanoma. Australas J Dermatol. 2020;61:179-80
286. Chew T, Pannell M, Jeeves A. Focal hair re-pigmentation associated with melanoma of the scalp. ANZ J Surg. 2020;90:1175-6
287. Hasegawa T, Iino S, Kitakaze K, Kato T, Kabata D, Oyama N. et al. Repigmentation of aging gray hair associated with unrecognized development and progression of amelanotic melanoma of the scalp: A physiological alert underlying hair rejuvenation. J Dermatol. 2021;48:e281-e3
288. Paus R. A neuroendocrinological perspective on human hair follicle pigmentation. Pigment Cell Melanoma Res. 2011;24:89-106
Author contact
![]() Corresponding author: Guan Jiang, Department of Dermatology, Affiliated Hospital of Xuzhou Medical College, Xuzhou 221002, China. Email: dr.guanjiangedu.cn.
Corresponding author: Guan Jiang, Department of Dermatology, Affiliated Hospital of Xuzhou Medical College, Xuzhou 221002, China. Email: dr.guanjiangedu.cn.
Received 2023-6-7
Accepted 2023-8-19
Published 2023-8-28
