ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2023; 19(14):4657-4671. doi:10.7150/ijbs.85767 This issue Cite
Research Paper
Pgam5-mediated PHB2 dephosphorylation contributes to endotoxemia-induced myocardial dysfunction by inhibiting mitophagy and the mitochondrial unfolded protein response
1. Department of Critical Care Medicine, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China.
2. Department of Critical Care Medicine, The First School of Clinical Medicine, Southern Medical University, Guangzhou 510515, China.
3. Department of Respiratory and Critical Care Medicine, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China.
4. Department of Pharmacy, Zhujiang Hospital, Southern Medical University, Guangzhou, 510280, China.
5. The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
6. School of Medicine, University of Rochester Medical Center Rochester, Rochester, NY 14642, United States.
7. Department of Cardiology, Huiqiao Medical Center, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China.
8. Senior Department of Cardiology, The Sixth Medical Center of People's Liberation Army General Hospital, Beijing, China.
*These authors contributed equally to this article.
Abstract
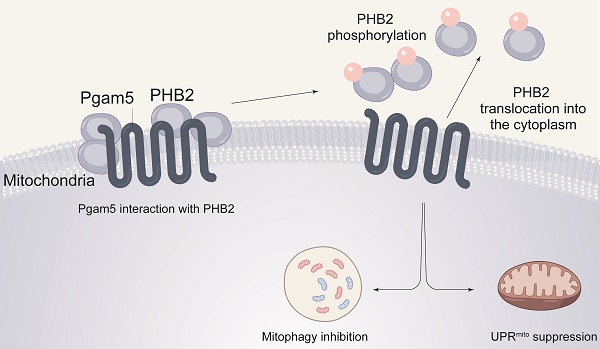
Numerous mitochondrial abnormalities are reported to result from excessive inflammation during endotoxemia. Prohibitin 2 (PHB2) and phosphoglycerate mutase 5 (Pgam5) have been associated with altered mitochondrial homeostasis in several cardiovascular diseases; however, their role in endotoxemia-related myocardial dysfunction has not been explored. Our experiments were aimed to evaluate the potential contribution of Pgam5 and PHB2 to endotoxemia-induced mitochondrial dysfunction in cardiomyocytes, with a focus on two endogenous protective programs that sustain mitochondrial integrity, namely mitophagy and the mitochondrial unfolded protein response (UPRmt). We found that PHB2 transgenic mice are resistant to endotoxemia-mediated myocardial depression and mitochondrial damage. Our assays indicated that PHB2 overexpression activates mitophagy and the UPRmt, which maintains mitochondrial metabolism, prevents oxidative stress injury, and enhances cardiomyocyte viability. Molecular analyses further showed that Pgam5 binds to and dephosphorylates PHB2, resulting in cytosolic translocation of mitochondrial PHB2. Silencing of Pgam5 or transfection of a phosphorylated PHB2 mutant in mouse HL-1 cardiomyocytes prevented the loss of mitochondrially-localized PHB2 and activated mitophagy and UPRmt in the presence of LPS. Notably, cardiomyocyte-specific deletion of Pgam5 in vivo attenuated LPS-mediated myocardial dysfunction and preserved cardiomyocyte viability. These findings suggest that Pgam5/PHB2 signaling and mitophagy/UPRmt are potential targets for the treatment of endotoxemia-related cardiac dysfunction.
Keywords: Pgam5, PHB2, endotoxemia-related cardiac dysfunction
