ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2023; 19(16):5292-5318. doi:10.7150/ijbs.89498 This issue Cite
Review
Protein arginine methylation in viral infection and antiviral immunity
1. School of Pharmacy, Shenzhen University Medical School, Shenzhen University, Shenzhen, 518055, China.
2. Institute of Biomedicine, College of Life Science and Technology, Guangdong Province Key Laboratory of Bioengineering Medicine, Key Laboratory of Innovative Technology Research on Natural Products and Cosmetics Raw Materials, Jinan University, Guangzhou, 510632, China.
Abstract
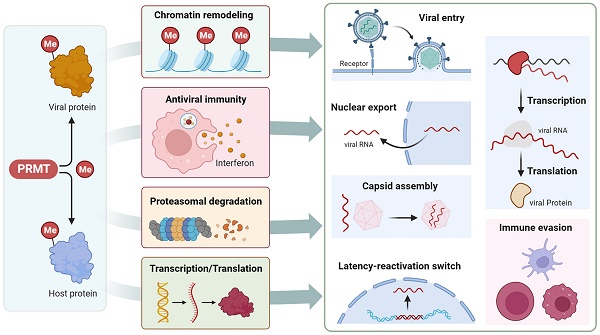
Protein arginine methyltransferase (PRMT)-mediated arginine methylation is an important post-transcriptional modification that regulates various cellular processes including epigenetic gene regulation, genome stability maintenance, RNA metabolism, and stress-responsive signal transduction. The varying substrates and biological functions of arginine methylation in cancer and neurological diseases have been extensively discussed, providing a rationale for targeting PRMTs in clinical applications. An increasing number of studies have demonstrated an interplay between arginine methylation and viral infections. PRMTs have been found to methylate and regulate several host cell proteins and different functional types of viral proteins, such as viral capsids, mRNA exporters, transcription factors, and latency regulators. This modulation affects their activity, subcellular localization, protein-nucleic acid and protein-protein interactions, ultimately impacting their roles in various virus-associated processes. In this review, we discuss the classification, structure, and regulation of PRMTs and their pleiotropic biological functions through the methylation of histones and non-histones. Additionally, we summarize the broad spectrum of PRMT substrates and explore their intricate effects on various viral infection processes and antiviral innate immunity. Thus, comprehending the regulation of arginine methylation provides a critical foundation for understanding the pathogenesis of viral diseases and uncovering opportunities for antiviral therapy.
Keywords: Protein arginine methyltransferase, antiviral immunity, arginine methylation, post-translational modifications, viral infection.
